Research Progress on Osteogenic Differentiation of Apical Papilla Stem Cells
-
摘要: 根尖牙乳头干细胞(stem cells from apical papilla,SCAP)具有很强的多系分化潜能,其中成骨分化可以应用于骨组织再生,为口腔颌骨缺损治疗提供新思路。成骨分化是个复杂的网络调控过程,诸如各种细胞因子、表观遗传物质、各种信号分子和信号通路等内源性物质均可产生不同程度的影响。这些因素相互作用可以促进SCAP的增殖、迁移和成骨分化,但其在SCAP成骨分化的不同进程中的具体机制和内在联系各不相同。对近年来有关促进SCAP成骨分化的各种因素及其可能的调控机制研究文献进行综述,以期为其进一步的应用研究提供新信息。Abstract: Stem cells from apical papilla (SCAP) have a strong multi-line differentiation potential, in which osteogenic differentiation can be applied to bone tissue regeneration, providing a new idea for the treatment of oral jaw defects. Osteogenic differentiation is a complex network regulation process, and endogenous substances such as various cytokines, epigenetic material, various signaling molecules and signaling pathways can have different degrees of influence. The interaction of these factors can promote the proliferation, migration and osteogenic differentiation of SCAP, but the specific mechanisms and internal links in different processes of osteogenic differentiation of SCAP are different. In this paper, the factors that promote osteogenic differentiation of SCAP and their possible regulatory mechanisms were reviewed to provide new information for further application research.
-
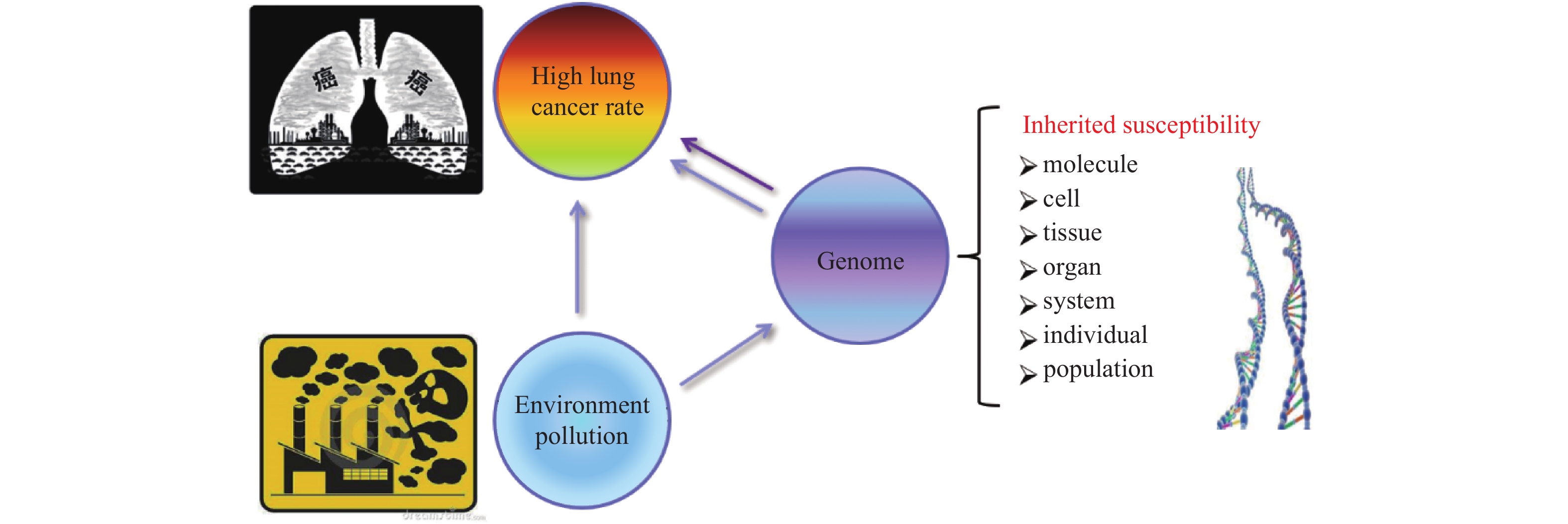
肺癌是发病率与死亡率最高的恶性肿瘤,严重威胁人类健康。肺癌的发生是环境与遗传因素相互作用的结果,环境因素是肺癌发生的诱因,遗传因素在肺癌发生、发展过程中起重要作用。携带遗传易感变异的个体,对环境中的致癌因素更为敏感,在环境与基因的相互作用中更易患癌。肺癌的发生发展涉及多基因参与、多阶段发展,伴随着大量基因的异常表达,相互作用形成复杂的分子网络,共同调控肿瘤细胞的生物学过程,从而导致肿瘤浸润、侵袭、转移等[1]。
云南省宣威地区(泛指珠江源地区,包括宣威市、富源县、麒麟县、会泽县、马龙县、沾益县及昭通与贵州西南部分区域),位于中国西南部,人口超过800万,2013年的最新调查数据显示,宣威地区男性肺癌发病率98.10/10万,是全国男性肺癌的2.37倍;女性83.22/10万,是全国女性肺癌的4.19倍,是全球女性肺癌发病率最高的地区[2-4]。该区域居民数百年来以烟煤作为主要生活能源,流行病学和实验研究表明:使用当地烟煤燃烧相关空气污染是该地区癌症风险和死亡率升高的原因[4-5]。
宣威地区肺癌高发并以家系为单位呈现出家族聚集现象,特别是给个人及家庭造成了巨大的经济压力和心理负担。以云南宣威家族肺癌为对象,研究空气污染、遗传易感性与肺癌发生的遗传学特征具有重要价值。
1. 空气污染与宣威家族肺癌聚集
1.1 环境致癌物与宣威肺癌
全球范围内约50%的人口以燃煤等生物燃料作为主要生活能源,生物燃料空气污染引发的健康问题日益突出。空气污染成分繁多,主要包括细颗粒物(PM2.5)、多环芳烃(polycyclic aromatic hydrocarbons,PAHs)、纳米级二氧化硅、砷、重金属、氡等,而多环芳烃是肺癌发生的主要原因之一。
中国约81%的人口在PM 2.5和家庭固体燃料污染超过WHO标准的环境中生活,空气污染是肺癌最重要的风险因素。不同地区空气污染成分差别较大,而城市空气污染PM2.5中含有多环芳烃。宣威地区居民世代在燃煤空气污染环境中生活繁衍,特殊的环境影响并塑造了当地人群独特的遗传背景,如图1。烟煤燃烧导致当地室内外空气中多环芳烃类化合物浓度居高不下,居民的生活环境相对封闭,长期持续暴露于PAHs浓度较高的环境中,其致癌因素也较为单一[6-8]。
黄云超与田林玮团队研究发现[9],宣威地区烟煤不完全燃烧释放出的多环芳烃(PAHs)、纳米二氧化硅、重金属、NO2等致癌物,以固体状细颗粒物(PM2.5)与凝结气溶胶形态散布于当地室内外空气中,通过呼吸道进入机体诱发癌变,是导致宣威地区人群肺癌高发主要原因。
Lan等[4]对1015名从不吸烟的女性和485名对照的综合暴露评估模型进行预测,分析了肺癌发病率与煤炭相关污染物暴露的关系,发现,PAH簇中的一组25种多环芳烃(PAHs)关联最强,其中以一类名为5-甲基䓛(5-methylchrysene)的亚种的关联性最强。
上世80年代末,云南省政府在宣威地区进行了部分改炉改灶工作,希望通过减少室内燃煤空气污染而降低肺癌发病率,燃煤炉灶的改造措施在一些局部地区有降低肺癌发病率的效果。但根据全国第3次死因调查结果提示:宣威地区肺癌发生率与死亡率仍是其它地区的3.3倍,且上升趋势明显,肺癌发病年龄呈现明显年轻化[10-12]。
2009 年云南省宣威地区被卫生部列为“农村高发肺癌早诊早治”工作试点,同时被纳入国家重点研发计划“肺癌高发现场高危人群队列”研究计划,宣威当地肺癌流行病学的现场调查中发现,宣威地区肺癌发病的家族聚集现象明显,早期 迁出宣威地区的家族肺癌人群也有较高肺癌发病率。
1.2 宣威家族肺癌发病规律
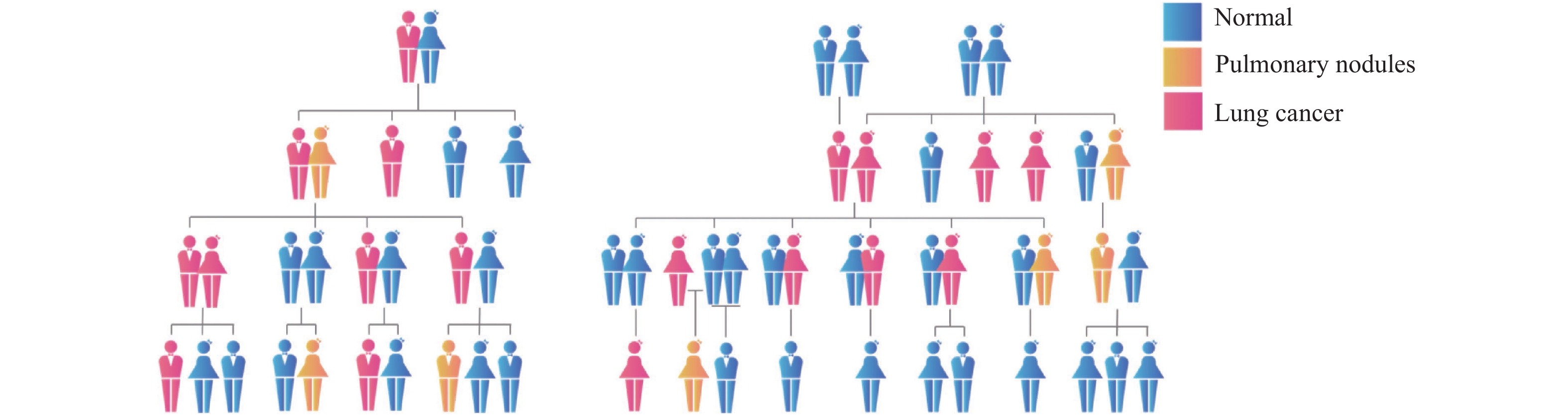
肺癌家系存在相似的发病规律:肺癌家族中几乎每一代中都有肺癌患者,大多为直系血亲,普遍呈现低年龄早发趋势,表现出明显的性别趋向。如图2所示,大多数家系中的肺癌患者以女性为主,并且连续2~3代,每一代中以女性患者多见,常见多个姐妹依年龄长幼顺序发病。少数家系则以男性肺癌患者为主,跨度为2~3代,每一代无或极少出现女性患者;对典型家族肺癌进行低剂量计算机断层扫描(low-dose computed tomography,LDCT)普查可发现:家族成员部分患者存在需定期随访的肺部结节。虽然同一家庭的成员其生活环境较为相似,行为和生活习惯也有较高的相似度;但是仅共享相似的生活环境和行为方式,还不足以完全解释宣威肺癌发病率的持续快速升高。
何兴舟等[13-14]对370个宣威家系分析结果表明,肺癌的发生具有家族聚集性,肺癌先证者的亲属(父母、兄弟姐妹)患肺癌的危险性增加,是配偶家系亲属(父母、兄弟、姐妹)的1.85倍,尤其是女性亲属,是配偶家系女性亲属的2.64倍,说明了遗传因素是肺癌的危险因素之一。环境中致癌物暴露并不是宣威地区肺癌发病率长期居高不下的唯一因素,肺癌遗传易感因素被严重低估。宣威家系肺癌这一特殊病人群体可能存在其特殊的发病机制,寻找其遗传易感相关的变异或分子特征,对肺癌的预防、诊断及治疗意义重大。
2. 宣威家族肺癌的临床特征与驱动基因突变谱
在长期燃煤空气污染:细颗粒物(PM2.5)、多环芳烃(PAHs)、纳米SiO2、重金属及Berthierine等暴露下,宣威地区人群的基因突变在多代繁衍中不断重塑,形成了稳定的遗传变异,导致宣威人群基因组的变异类型、分布、基因组稳定性等特征异于普通人群[9, 15-18]。
这类与环境致癌交互作用导致的遗传变异在肺癌家族人群中的富集度更高,直接或间接影响并塑造了家族肺癌患者独特的临床病理表型和遗传背景。
2.1 宣威地区家族肺癌患者的临床病理特征
近10 a来,随着肺癌诊断水平和居民医疗条件及健康意识的快速提升,我国肺癌的流行病学和临床病理特征结构已发生巨大改变:女性、非吸烟、腺癌及肺癌家族史的患者占比显著增加,鳞癌显著减少。特别是LDCT 在肺癌筛查及健康体检中的广泛运用,大量低龄、以肺部多发结节为表现的腺癌患者出现,女性与家族肺癌史患者占比高,其中相当比例肺结节患者为多原发肺癌(multiple primary lung cancer,MPLC)[19-21]。
Ding等[19-21]对宣威家族肺癌患者的临床特征及疾病谱进行了分析发现,与散发肺癌人群相比,宣威地区有家族聚集性肺癌史的患者发病年龄提前10~15 a,其中女性发病年龄提前更为明显。家族中多代人群均存在不同程度的燃煤空气暴露;患者中女性占比高,病理类型以非鳞癌多见,易较早发生远处转移;双肺多灶性病变(MPLC)比例较高,手术切除率低,遗传因素显著提高了宣威地区肺癌的发病风险。
MPLC患者的临床特征与宣威家族肺癌患者的临床特点极为相似:低龄化,腺癌、女性占比高,EGFR突变检出率高,且MPLC患者多数有家族肺癌史[22-23]。
家族肺癌患者中合并有高血压、糖尿病及其他癌症(甲状腺癌与结直肠癌)的患者占比高;更易较早发生如息肉、结节及囊肿等病变。Lan等[20]研究也发现宣威肺癌患者的心脑血管意外发生率与烟煤暴露程度呈正相关。
2.2 宣威地区家族肺癌患者驱动基因突变谱与燃煤暴露关系
Chen等[5, 19]研究发现,宣威地区家族肺癌患者的驱动基因突变与燃煤暴露情况进行评估发现:宣威地区家族肺癌患者驱动基因谱独特,且与燃煤暴露程度密切相关,宣威地区患者ALK、ROS1等基因融合与女性、非吸烟、低龄,肺癌家族史与燃煤暴露密切相关;宣威家族肺癌患者EGFR基因谱中G719X、G719S与S768I等罕见突变占比较高,而 Del 19与 L858R等常见突变占比低。燃煤暴露程度与肺癌家族史是导致宣威地区肺癌驱动基因谱与散发肺癌患者差异显著的重要因素[24]。
3. 宣威肺癌的遗传易感研究
宣威地区肺癌发病的家族聚集现象,给家族成员带来了巨大的心理压力。特别是对体检发现肺部小结节的人群而言,焦虑与恐惧情绪严重,过度检查等事件时有发生,严重影响了当地居民的正常生活[25-26]。
肺癌是环境与遗传交互作用的结果,癌变相关的遗传易感特征涉及基因变异、基因转录、蛋白表达及机体微生态等多因素的相互作用。肺癌的基因易感性主要涉及:代谢酶基因多态性、抑癌基因多态性、DNA 修复酶基因多态性、非编码RNA调控紊乱,以及某些基因的突变缺失[27-28]。
3.1 单核苷酸多态性与家族肺癌遗传易感性
染色体DNA变异最多见的是单核苷酸多态性(SNP),全基因组关联研究(GWAS)是分析鉴定疾病相关SNP位点的经典方法[29]。全基因组关联研究(GWAS)发现:TERT基因的多个SNP位点与肺癌的遗传易感性密切相关[30]。美国国立癌症研究所(NCI)Lan等[31-32]研究也证实,TERT的SNP位点rs2075786、rs560191与肺癌易感性明显相关,TT基因型非小细胞肺癌患者生存率明显降低,但TERT并非通过影响端粒长度发挥遗传易感作用。肺癌易感基因可能会影响 TERT的表达,使细胞易于逃过凋亡而永生化,导致肺癌的发生。
3.2 家族肺癌遗传易感相关蛋白编码基因
Cao等[15]通过对宣威地区9名典型家肺癌族成员的全基因组测序(whole genome sequencing,WGS)的分析结果发现, ARHGEF5、ANKRD20A2、ZNF595、ZNF812、MYO18B基因突变可能是肺癌潜在的胚系突变。对12个基因内的特异性突变,发现:MUC16、MUC12、FOXD4L3和FOXD4L5基因内的突变频率更高,这些基因的拷贝数变异可以潜在地与易患肺癌相关。对宣威家族肺癌的WGS数据分析发现:非编码DNA变异可能改变了系统的分析和配置。胚系突变也可发生在非编码RNA。这项研究为宣威家族肺癌的突变谱和遗传易感性评价提供了重要线索。
Chen等[33]研究发现,MUC16在宣威肺癌中高表达且DNA区变异频率高,MUC16表达与肺癌家族史、室内燃煤暴露及肺癌分期相关。在部分高表达MUC16的肺癌患者呈现明显偏低的外周血淋巴细胞计数,推测癌细胞可能通过上调MUC16干扰人体免疫系统进而保护肿瘤,提示MUC16促癌功能的多样性。在肺癌细胞系H23、H838中过表达MUC16可提高细胞的增殖能力、迁移侵袭能力以及对顺铂、紫衫醇类化疗药物的耐药性[34]。
3.3 LncRNA与宣威家族肺癌遗传易感
宣威地区家族肺癌人群的全基因组测序,在不同独立家系的变异交集和变异并集中,筛选出变异密度高的基因或染色体区域分析发现:大多数高变异密度的区域分布于基因间区,部分基因表现出显著高于周边区域的变异密度,其中包含大量非编码RNA[35],见图3。
家族肺癌患者的全基因组测序数据涵盖了大量的遗传特征,通过分析筛选出的基因或染色体区域,可能直接或间接与肺癌遗传易感性相关,也可能只反映家族肺癌人群的其他生理功能。这些与肺癌易感直接或间接相关的特征,在宣威地区独特的环境和生活方式类似的家族中高度富集。
宣威肺癌家系基因组中交集分析突变频率前十的LncRNA提示:Linc00665、Linc00624、Linc00320、Linc00842可能与非小细胞肺癌发生发展有关。LINC00842在无淋巴结转移的患者中表达水平较高,发生淋巴结转移的患者,血细胞LINC00842表达量普遍偏低,LINC00842在吸烟患者群体中更倾向于高表达,在非吸烟患者中,血细胞LINC00842表达量偏低。LINC00433在MPLC患者中血细胞低表达,尤其在大于55岁男性患者更为显著[35]。Benzopyrene处理30 d后Q-PCR检测原LncRNA的表达水平未发生显著波动,再次检测16HBE的增殖和迁移能力,发现:LINC00343和LINC00433过表达的16HBE细胞系经过致癌物诱变后增殖和运动能力有所提高,但是干扰这2个LncRNA并没有观察到明显效应,推测LINC00343和LINC00433能够在一定程度上影响细胞系对致癌诱变剂的敏感性[35-36]。
3.4 肺部微生态对宣威家族肺癌遗传易感的影响
人体内的微生物组在肺癌的发生发展中起着核心作用,遗传可能通过影响宿主代谢进而造成菌群组成的差异,微生分类群的丰度受宿主遗传因素的影响[37-39]。烟煤燃烧释放的PAHs是宣威地区生态环境中主要的致癌物之一,PAHs难以在短期内以生物降解形式消除,在环境中长期滞留可导致降解PAHs的鞘氨醇单胞菌、苍白杆菌丰度显著增加[40]。
Hosgood等[40-42]对宣威地区女性肺癌患者的痰液微生物菌群检查发现,颗粒链菌属、营养缺陷菌属以及链球菌属的相对丰度明显高于正常人,并推测呼吸道微生物可能影响了多环芳烃(PAHs)等致癌物在体内的代谢,参与了肺癌的发生。宣威地区家族肺癌组织中也发现:具备降解PAHs能力的菌属的鞘氨醇单胞菌、苍白杆菌丰度显著增加,大肠杆菌的相对丰度也有增加。
宣威当地燃煤暴露影响了肺部微生物菌群的构成,家族患者的遗传背景可能通过改变支气管粘液层的特性,进而利于降解PAHs的特殊菌种在肺部定殖。环境暴露、肺部微生态与遗传变异交互作用可能影响肺癌易感性,但具体机制有待进一步研究。
4. 小结
宣威地区肺癌的高发与室内燃煤产物直接相关。肺癌大流行具有明显的家族聚集性,遗传变异在宣威地区肺癌的发生中发挥重要作用。尽管已经发现了许多与肺癌相关的基因突变,但宣威地区肺癌的潜在遗传易感机制相当复杂,仍未完全阐明。因此,宣威人群肺癌发生和发展的机制有待进一步研究。对宣威家族肺癌遗传变异与遗传易感机制的深入研究,可为肺癌易感人群的筛选、早期干预及风险评估提供帮助。
-
[1] Morsczeck C. Dental stem cells for tooth regeneration: how far have we come and where next?[J]. Expert Opin Biol Ther,2023,23(6):527-537. doi: 10.1080/14712598.2023.2208268 [2] Sonoyama W,Liu Y,Fang D,et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine[J]. PLoS One,2006,1(1):e79-86. doi: 10.1371/journal.pone.0000079 [3] Liu Q,Gao Y,He J. Stem cells from the apical papilla (SCAPs): past,present,prospects,and challenges[J]. Biomedicines,2023,11(7):2047-2059. doi: 10.3390/biomedicines11072047 [4] Bakopoulou A,Leyhausen G,Volk J,et al. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP)[J]. Arch Oral Biol,2011,56(7):709-721. doi: 10.1016/j.archoralbio.2010.12.008 [5] Chen K,Xiong H,Huang Y,et al. Comparative analysis of in vitro periodontal characteristics of stem cells from apical papilla (SCAP) and periodontal ligament stem cells (PDLSCs)[J]. Arch Oral Biol,2013,58(8):997-1006. doi: 10.1016/j.archoralbio.2013.02.010 [6] Chang H H,Chang M C,Wu I H,et al. Role of ALK5/Smad2/3 and MEK1/ERK signaling in transforming growth factor beta 1-modulated growth,collagen turnover,and differentiation of stem cells from apical papilla of human tooth[J]. J Endod,2015,41(8):1272-1280. doi: 10.1016/j.joen.2015.03.022 [7] Li J,Ge L,Zhao Y,et al. TGF-beta2 and TGF-beta1 differentially regulate the odontogenic and osteogenic differentiation of mesenchymal stem cells[J]. Arch Oral Biol,2022,64(3):105357. [8] Yu S,Li J,Zhao Y,et al. Comparative secretome analysis of mesenchymal stem cells from dental apical papilla and bone marrow during early odonto/osteogenic differentiation: potential role of transforming growth factor-beta2[J]. Front Physiol,2020,11(3):41-53. [9] Zhang W,Zhang X,Ling J,et al. Proliferation and odontogenic differentiation of BMP2 genetransfected stem cells from human tooth apical papilla: an in vitro study[J]. Int J Mol Med,2014,34(4):1004-1012. doi: 10.3892/ijmm.2014.1862 [10] Zhang W,Zhang X,Li J,et al. Foxc2 and BMP2 induce osteogenic/odontogenic differentiation and mineralization of human stem cells from apical papilla[J]. Stem Cells Int,2018,9(7):2363917. [11] Zhang W,Zhang X,Ling J,et al. Osteo-/odontogenic differentiation of BMP2 and VEGF gene-co-transfected human stem cells from apical papilla[J]. Mol Med Rep,2016,13(5):3747-3754. doi: 10.3892/mmr.2016.4993 [12] Press T,Viale-Bouroncle S,Felthaus O,et al. EGR1 supports the osteogenic differentiation of dental stem cells[J]. Int Endod J,2015,48(2):185-192. doi: 10.1111/iej.12299 [13] Wang J,Zhang H,Zhang W,et al. Bone morphogenetic protein-9 effectively induces osteo/odontoblastic differentiation of the reversibly immortalized stem cells of dental apical papilla[J]. Stem Cells Dev,2014,23(12):1405-1416. doi: 10.1089/scd.2013.0580 [14] Wang F,Jiang Y,Huang X,et al. Pro-Inflammatory cytokine TNF-alpha attenuates BMP9-induced osteo/ odontoblastic differentiation of the stem cells of dental apical papilla (SCAPs)[J]. Cell Physiol Biochem,2017,41(5):1725-1735. doi: 10.1159/000471865 [15] Zhang H,Wang J,Deng F,et al. Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs)[J]. Biomaterials,2015,39(1):145-154. [16] Zhu X Y,Diao S,Yang D M,et al. The Mechanism of GREM1's effect on osteogenic/odontogenic differentiation of stem cells from apical papilla[J]. Sichuan Da Xue Xue Bao Yi Xue Ban,2021,52(3):409-415. [17] Wang S,Mu J,Fan Z,et al. Insulin-like growth factor 1 can promote the osteogenic differentiation and osteogenesis of stem cells from apical papilla[J]. Stem Cell Res,2012,8(3):346-356. doi: 10.1016/j.scr.2011.12.005 [18] Wang Y,Pang X,Wu J,et al. MicroRNA hsa-let-7b suppresses the odonto/osteogenic differentiation capacity of stem cells from apical papilla by targeting MMP1[J]. J Cell Biochem,2018,119(8):6545-6554. doi: 10.1002/jcb.26737 [19] Ma S,Liu G,Jin L,et al. IGF-1/IGF-1R/hsa-let-7c axis regulates the committed differentiation of stem cells from apical papilla[J]. Sci Rep,2016,6(11):36922-36933. [20] Cao Y,Xia D S,Qi S R,et al. Epiregulin can promote proliferation of stem cells from the dental apical papilla via MEK/Erk and JNK signalling pathways[J]. Cell Prolif,2013,46(4):447-456. doi: 10.1111/cpr.12039 [21] Li Y,Yan M,Wang Z,et al. 17beta-estradiol promotes the odonto/osteogenic differentiation of stem cells from apical papilla via mitogen-activated protein kinase pathway[J]. Stem Cell Res Ther,2014,5(6):125-149. doi: 10.1186/scrt515 [22] Wang Y,Lu Y,Li Z,et al. Oestrogen receptor alpha regulates the odonto/osteogenic differentiation of stem cells from apical papilla via ERK and JNK MAPK pathways[J]. Cell Prolif,2018,51(6):e12485-12494. doi: 10.1111/cpr.12485 [23] Pang X,Zhuang Y,Li Z,et al. Intermittent administration of parathyroid hormone enhances odonto/osteogenic differentiation of stem cells from the apical papilla via JNK and P38 MAPK pathways[J]. Stem Cells Int,2020,11(2):5128128. [24] Zhang J,Zhao I S,Yu O Y,et al. Layer-by-layer self-assembly polyelectrolytes loaded with cyclic adenosine monophosphate enhances the osteo/odontogenic differentiation of stem cells from apical papilla[J]. J Biomed Mater Res A,2021,109(2):207-218. doi: 10.1002/jbm.a.37017 [25] Su S,Zhu Y,Li S,et al. The transcription factor cyclic adenosine 3',5'-monophosphate response element-binding protein enhances the odonto/osteogenic differentiation of stem cells from the apical papilla[J]. Int Endod J,2017,50(9):885-894. doi: 10.1111/iej.12709 [26] Zhang Y,Yuan L,Meng L,et al. Guanine and nucleotide binding protein 3 promotes odonto/osteogenic differentiation of apical papilla stem cells via JNK and ERK signaling pathways[J]. Int J Mol Med,2019,43(1):382-392. [27] Xiao M,Yao B,Zhang B D,et al. Stromal-derived Factor-1alpha signaling is involved in bone morphogenetic protein-2-induced odontogenic differentiation of stem cells from apical papilla via the Smad and Erk signaling pathways[J]. Exp Cell Res,2019,381(1):39-49. doi: 10.1016/j.yexcr.2019.04.036 [28] Liu J,Wang X,Song M,et al. MiR-497-5p regulates osteo/odontogenic differentiation of stem cells from apical papilla via the smad signaling pathway by targeting smurf2[J]. Front Genet,2020,11(10):582366. [29] Li Z,Ge X,Lu J,et al. MiR-141-3p regulates proliferation and senescence of stem cells from apical papilla by targeting YAP[J]. Exp Cell Res,2019,383(2):111562. doi: 10.1016/j.yexcr.2019.111562 [30] Xiao Y,Chen L,Xu Y,et al. Circ-ZNF236 mediates stem cells from apical papilla differentiation by regulating LGR4-induced autophagy[J]. Int Endod J,2024,57(4):431-450. doi: 10.1111/iej.14021 [31] Jia Q,Chen X,Jiang W,et al. The regulatory effects of long noncoding RNA-ANCR on dental tissue-derived stem cells[J]. Stem Cells Int,2016,7(8):3146805. [32] Wang L,Yang H,Lin X,et al. KDM1A regulated the osteo/dentinogenic differentiation process of the stem cells of the apical papilla via binding with PLOD2[J]. Cell Prolif,2018,51(4):e12459-12467. doi: 10.1111/cpr.12459 [33] Diao S,Yang D M,Dong R,et al. Enriched trimethylation of lysine 4 of histone H3 of WDR63 enhanced osteogenic differentiation potentials of stem cells from apical papilla[J]. J Endod,2015,41(2):205-211. doi: 10.1016/j.joen.2014.09.027 [34] Gao R,Dong R,Du J,et al. Depletion of histone demethylase KDM2A inhibited cell proliferation of stem cells from apical papilla by de-repression of p15INK4B and p27Kip1[J]. Mol Cell Biochem,2013,379(1-2):115-122. doi: 10.1007/s11010-013-1633-7 [35] Su X,Yang H,Shi R,et al. Depletion of SNRNP200 inhibits the osteo-/dentinogenic differentiation and cell proliferation potential of stem cells from the apical papilla[J]. BMC Dev Biol,2020,20(1):22-32. doi: 10.1186/s12861-020-00228-y [36] Xu J,Yu B,Hong C,et al. KDM6B epigenetically regulates odontogenic differentiation of dental mesenchymal stem cells[J]. Int J Oral Sci,2013,5(4):200-205. doi: 10.1038/ijos.2013.77 [37] Li W,Lin X,Yang H,et al. Depletion of HOXA5 inhibits the osteogenic differentiation and proliferation potential of stem cells from the apical papilla[J]. Cell Biol Int,2018,42(1):45-52. doi: 10.1002/cbin.10860 [38] Gao R T,Zhan L P,Meng C,et al. Homeobox B7 promotes the osteogenic differentiation potential of mesenchymal stem cells by activating RUNX2 and transcript of BSP[J]. Int J Clin Exp Med,2015,8(7):10459-10470. [39] Yang H,Liang Y,Cao Y,et al. Homeobox C8 inhibited the osteo-/dentinogenic differentiation and migration ability of stem cells of the apical papilla via activating KDM1A[J]. J Cell Physiol,2020,235(11):8432-8445. doi: 10.1002/jcp.29687 [40] Wu Z,Wang J,Dong R,et al. Depletion of MEIS2 inhibits osteogenic differentiation potential of human dental stem cells[J]. Int J Clin Exp Med,2015,8(5):7220-7230. [41] Yang H,Fan J,Cao Y,et al. Distal-less homeobox 5 promotes the osteo-/dentinogenic differentiation potential of stem cells from apical papilla by activating histone demethylase KDM4B through a positive feedback mechanism[J]. Exp Cell Res,2019,374(1):221-230. doi: 10.1016/j.yexcr.2018.11.027 [42] Yang H,Cao Y,Zhang J,et al. DLX5 and HOXC8 enhance the chondrogenic differentiation potential of stem cells from apical papilla via LINC01013[J]. Stem Cell Res Ther,2020,11(1):271-286. doi: 10.1186/s13287-020-01791-8 [43] Wan F,Gao L,Lu Y,et al. Proliferation and osteo/odontogenic differentiation of stem cells from apical papilla regulated by Zinc fingers and homeoboxes 2: An in vitro study[J]. Biochem Biophys Res Commun,2016,469(3):599-605. doi: 10.1016/j.bbrc.2015.11.135 [44] Zhang J,Wang Z,Jiang Y,et al. Nuclear Factor I-C promotes proliferation and differentiation of apical papilla-derived human stem cells in vitro[J]. Exp Cell Res,2015,332(2):259-266. doi: 10.1016/j.yexcr.2015.01.020 [45] Wang H,Cao Y. WIF1 enhanced dentinogenic differentiation in stem cells from apical papilla[J]. BMC Oral Health,2019,19(1):25-32. doi: 10.1186/s12903-018-0700-6 [46] Jin L,Cao Y,Yu G,et al. SFRP2 enhances the osteogenic differentiation of apical papilla stem cells by antagonizing the canonical WNT pathway[J]. Cell Mol Biol Lett,2017,22(8):14-27. [47] Yang H,Li G,Han N,et al. Secreted frizzled-related protein 2 promotes the osteo/odontogenic differentiation and paracrine potentials of stem cells from apical papilla under inflammation and hypoxia conditions[J]. Cell Prolif,2020,53(1):e12694-12704. doi: 10.1111/cpr.12694 [48] Zhou M,Guo S,Yuan L,et al. Blockade of LGR4 inhibits proliferation and odonto/osteogenic differentiation of stem cells from apical papillae[J]. J Mol Histol,2017,48(5-6):389-401. doi: 10.1007/s10735-017-9737-0 [49] Cheng Q,Zeng K,Kang Q,et al. The antimicrobial peptide LL-37 promotes migration and odonto/osteogenic differentiation of stem cells from the apical papilla through the Akt/Wnt/beta-catenin signaling pathway[J]. J Endod,2020,46(7):964-972. doi: 10.1016/j.joen.2020.03.013 [50] Liu J,Du J,Chen X,et al. The effects of mitogen-activated protein kinase signaling pathways on lipopolysaccharide-mediated osteo/odontogenic differentiation of stem cells from the apical papilla[J]. J Endod,2019,45(2):161-167. doi: 10.1016/j.joen.2018.10.009 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 826
- HTML全文浏览量: 658
- PDF下载量: 19
- 被引次数: 0





 下载:
下载:

 下载:
下载:
