The Distribution Characteristics of Pathogenic Bacteria in Inpatients with Diabetic Foot were Correlated with Different Wagner Grades
-
摘要:
目的 研究云南省第三人民医院住院糖尿病足患者病原菌感染情况及与不同Wagner分级的相关性,了解糖尿病足住院患者感染病原菌特点及相关危险影响因素,进一步为糖尿病足患者抗感染治疗提供理论指导。 方法 回顾分析云南省第三人民医院2019年1月至2023年1月检测出细菌感染的536例糖尿病足患者的人口数据学资料、足溃疡严重程度、相关实验室检查结果。 结果 共536例糖尿病足患者培养出病原菌,其中感染革兰氏阳性菌268例(50.0%)、感染革兰氏阴性菌214例(39.9%)、感染真菌2例(0.4%)及感染混合细菌52例(9.7%)。感染革兰氏阳性菌中以金黄色葡萄球菌、表皮葡萄球菌、粪肠球菌为主要病原菌;革兰阴性菌以大肠埃希菌、阴沟肠杆菌、肺炎克雷伯杆菌为主。共有31例多重耐药菌,多重耐药率为(5.78%),其中革兰氏阳性菌中多重耐药均为金黄色葡萄球菌,革兰氏阴性菌中多重耐药菌为鲍曼不动杆菌(1例)、肺炎克雷伯杆菌(2例)、普通变形杆菌(2例)、铜绿假单胞菌(5例)、奇异变形菌(1例)、阴沟肠杆菌(1例)。将536例患者分为Wagner1、2级组 (78 例 )、Wagner3 级组 (274 例)和 Wagner4、5级组 (184 例 )。Wagner1、2级组感染单一细菌73例,混合细菌5 例,其中包括革兰阳性菌51例(65.4%),革兰阴性菌21例(26.9%),真菌1例(1.3%);Wagner3级组感染单一细菌248例,混合细菌26例。其中感染革兰阳性菌144例(52.6%),革兰阴性菌103例(37.6%),真菌1例(0.4%)。Wagner4、5级组感染单一细菌163例,混合细菌21例。其中感染革兰阳性菌73株(39.7%),革兰阴性菌90株(48.9%),真菌0株(0%)。Wagner1、2、3级患者感染病原菌以革兰阳性菌为主,Wagner4、5级感染病原菌以革兰阴性菌为主。不同Wagner分级的糖尿病足患者的白细胞计数、中性粒细胞百分比、细菌分类情况、住院天数、血沉、白蛋白差异具有统计学意义 (P < 0.01),随着Wagner分级增高,患者白细胞计数、超敏C反应蛋白更高,住院天数更长,白蛋白水平更低;在年龄、性别、糖尿病病程、有无吸烟史、有无饮酒史、有无高血压病病史之间差异无统计学意义 (P > 0.05)。 结论 糖尿病足溃疡患者感染细菌情况与不同Wagner分级有关,Wagner分级越高,感染革兰氏阴性菌可能性越大,入院时可根据患者Wagner分级情况合理选择抗生素,积极控制感染,同时加强营养、缩短住院天数,减少截肢率发生,从而改善糖尿病足患者的预后。 Abstract:Objective To study the pathogenic bacteria infection in hospitalized diabetic foot patients in the Third People's Hospital of Yunnan Province and its correlation with different Wagner grades, to understand the the characteristics of pathogenic bacteria and related risk factors in hospitalized diabetic foot patients in the Third People's Hospital of Yunnan Province, and to further provide theoretical guidance for anti-infection treatment of these patients. Methods A retrospective analysis was conducted on the demographic data, severity of foot ulcers, and related laboratory test results of 536 patients with diabetic foot who were detected to have bacterial infection in the Third People's Hospital of Yunnan Province from January 2019 to January 2023. Results Among the 536 diabetic foot patients, pathogenic bacteria were cultured from 268 cases (50.0%) of Gram-positive bacterial infections, 214 cases(39.9%) of gram-negative bacterial infections, 2 cases(0.4%) of fungal infections, and 52 cases (9.7%) of mixed bacterial infections. The main pathogens among gram-positive bacteria were Staphylococcus aureus, Staphylococcus epidermidis and Enterococcus faecalis. for Gram-negative bacteria, the main pathogens were Escherichia coli, Enterobacter cloacae and Klebsiella pneumoniae.There were 31 cases of multi-drug resistant bacteria, and the multi-drug resistance rate was (5.78%). Among Gram-positive bacteria, all multidrug-resistant strains were Staphylococcus aureus, while among Gram-negative bacteria, the multi-drug resistant strains included Acinetobacter baumannii (1 case), Klebsiella pneumoniae (2 cases), Proteus common (2 cases), Pseudomonas aeruginosa (5 cases), Proteus mirabilis (1 case) and Enterobacter cloacae (1 case). The 536 patients were divided into Wagner grade 1 and 2 groups (78 cases), Wagner grade 3 group (274 cases), and Wagner grade 4 and 5 groups (184 cases). There were 73 cases of single bacterial infections and 5 cases of mixed bacterial infections in Wagner grade 1 and 2 group, including 51 cases (65.4%) of gram-positive bacteria, 21 cases (26.9%) of gram-negative bacteria and 1 case (1.3%) of fungi. There were 248 cases of single bacterial infections and 26 cases of mixed bacterial infections in Wagner3 group, with 144 cases (52.6%) of gram-positive bacteria, 103 cases (37.6%) of gram-negative bacteria, and 1 case (0.4%) with fungi. In the Wagner grade 4 and 5 groups, there were 163 cases of single bacterial infections and 21 cases of mixed bacterial infection, with 73 strains( 39.7%) of gram-positive bacteria, 90 strains (48.9%) of gram-negative bacteria and 0 strain (0%) of fungi.The predominant infectious pathogens in Wagner grades 1, 2 and 3 were gram-positive bacteria, while those in Wagner grades 4 and 5 patients were mainly gram-negative bacteria. There were statistically significant differences in white blood cell counts, neutrophil percentage, bacterial classification, length of hospital stay, erythrocyte sedimentation rate and albumin levels among diabetic foot patients with different Wagner grades (P < 0.01). With the increase of Wagner grade, patients had higher white blood cell counts and hypersensitive C-reactive protein levels, longer hospital stays, and lower albumin levels; however, there were no statistically significant differences in age, sex, duration of diabetes, smoking history, alcohol consumption history and history of hypertension (P > 0.05). Conclusion The bacterial infection situation in patients with diabetic foot ulcers is related to different Wagner grades. The higher the Wagner grades, the greater the likelihood of infection with gram-negative bacteria. Antibiotics can be reasonably selected according to the Wagner grades of patients upon admission, actively controlling infection, while also enhancing, shortening hospital stays, and reducing amputation rates, thereby improving the prognosis of diabetic foot patients. -
Key words:
- Diabetes mellitus /
- Diabetic foot /
- Pathogenic bacteria /
- Wagner classification
-
难治性创面是指对初期治疗无反应或经护理但持续存在的伤口[1]。褥疮、糖尿病足、腹部巨大缺损的治疗手段有电刺激、辐射热、生长因子和皮肤等效物以及负压疗法[2]。持续负压吸引术是一种新型、高效的引流设备,用于治疗各种类型的难以愈合的伤口,与传统的引流设备相比,持续负压吸引术的疗效是显而易见的[3-4],用于修复复杂的创伤和大面积软组织缺损[5],负压环境中,血流量增加,肉芽组织形成更牢固,细菌清除率提高[6],而本研究中富氧负压辅助伤口疗法(regulated oxygen-enriched negative pressure-assisted wound therapy,RO-NPT)与单纯负压吸引治疗相比,有助于创面恢复和炎症控制[7]。Top Closure皮肤牵拉闭合器可持续使用[8],由以色列西勒雅法医学中心Moris Topaz教授首次运用于临床治疗难治性创面,该装置可实现大型软组织缺损一期闭合[9]。本文通过回顾性研究分析该装置治疗难治性创面的36例患者,探讨其治疗效果,以便于临床医生使用该装置作为参考依据。
1. 资料与方法
资料来源于昆明医科大学第一附属医院胃肠与疝外科2018年8月至2020年7月收治的难治性创面患者。
1.1 纳入标准
本研究纳入2018年8月至2020年7月在昆明医科大学第一附属医院接受皮肤牵拉闭合器联合富氧可调节负压辅助疗法的连续难治性创面患者[4],纳入研究对象包括:(1)患者年龄19~90岁;(2)患者皮肤和/或底层组织的局部损伤,确诊为褥疮;(3)患者因糖尿病血糖控制不佳,造成足部溃疡,诊断为糖尿病足;(4)患者因腹部疾病需要外科手术干预造成腹壁巨大缺损,不能用传统缝合技术缝合的腹部手术切口。本研究经昆明医科大学第一附属医院伦理审查委员会审核通过,所有纳入患者均签署知情同意书。
1.2 排除标准
(1)患者不愿意接受Top Closure皮肤牵拉闭合器联合RO-NPT治疗技术;(2)患者不能耐受手术,或者合并有患有人类免疫缺陷病毒,乙型肝炎病毒或丙型肝炎病毒阳性;(3)在60 d内接受免疫治疗或细胞毒性化疗的患者;(4)因任何原因被研究者视为不合适人选的患者。
1.3 病历资料
符合纳入标准的难治性创面患者36例,其中男20例,女16例,年龄19~90岁,中位年龄54.5岁。其中褥疮患者30例,创面面积3 cm×3 cm~8 cm×6 cm,骶尾部褥疮患者25例,臀部肛门旁褥疮患者5例;糖尿病足患者5例,创面大小2 cm×4 cm×2 cm~2 cm×3 cm×5 cm,创面均位于足踝;腹部巨大缺损患者1例,该例患者因为腹部肌层侵袭性纤维瘤病,手术切除腹壁肌层肿瘤后造成腹壁巨大缺损,创面大小为15 cm×3 cm。
1.4 方法
材料:Top Closure皮肤闭合牵张器(两片粘贴板:可采用自带双面胶粘贴固定或使用皮肤吻合器或缝合线来将之固定于正常组织表面;一根牵张条:可将两侧的粘贴板拉拢),一次性负压治疗吸附垫(包括医用PU海绵,引流吸盘,医用贴膜,隔离垫),负压治疗仪(可接医院中心负压,按需设定负压值,调节范围:0~300 mmHg。),所有材料均由云南昆明佰奥勒科技有限公司提供,生产批号:20191127。
皮肤持续牵张技术:根据手术原则,进行彻底清创,以创面为中心将坏死组织锐性切除(包括皮肤,皮下及坏死筋膜)直至创面见新鲜出血,使伤口空腔彻底开放,以便双氧水以及三型碘反复冲洗。于创面边缘正常皮肤安置适当数量的皮肤张力扩张器,初步拉和创面,张力中等;以减张线及皮缝器加强固定皮肤张力扩张器。创面内放置无菌海绵,通以氧管及冲洗管,创面外再以无菌海绵覆盖,表面覆以透明膜粘贴创面。
富氧负压辅助伤口疗法:通过侧管用无菌生理盐水缓慢持续地冲洗伤口,以保持敷料湿润。为了监测疗效并避免引流管阻塞,应注意敷料外观的任何变化或敷料下方的任何渗出物堆积(以及引流液的量和性质)。引流管堵塞时及时反复冲洗。如果发现血性液体,负压降低。如果引流液中仍有血迹或无法清除堵塞的引流管,则移除引流装置并进行严格的止血。期间密切关注手部和指尖的血液循环,如有必要,调整负压,或移除敷料。总的来说,褥疮的负压控制在70~120 mmHg之间,一般3~5 d更换一次敷料;糖尿病溃疡患者依据其分期调节负压,慢性期患者负压控制在50~70 mmHg之间,急性期患者负压控制在60~90 mmHg之间,均3~5 d更换一次敷料,尽可能使用较低的有效负压,当创面渐渐清洁后将压力调低。
1.5 疗效评估
疤痕质量:根据温哥华疤痕等级(vancouver scar scale,VSS)评估疤痕质量,包括疤痕的4个变量(色素沉着、血管性、柔韧性和高度),每个变量得分越高,说明疤痕越严重[10]。
治疗效果评估:痊愈:经过治疗后创面愈合,症状消失。显效:经过治疗后创面仍然存在一定程度的疼痛,而且创面愈合率达到70%以上。有效:经过治疗后创面疼痛可以得到有效减轻,而且创面愈合率在21%~69%之间。好转:经过治疗后创面疼痛未得到缓解,而且创面愈合率在20%以下。无效:治疗后创面的疼痛加重,创面面积未减少,甚至加大[11]。
连续的褥疮创面评估包括:褥疮愈合量表,褥疮状态工具,会话量表,本研究采用褥疮愈合量表作为评估方式。
1.6 统计学处理
所有数据采用SPSS 21.0进行统计分析,符合正态分布的数据以平均值±标准差(
$\bar x \pm s$ )表示。2. 结果
36例患者完成Top Closure皮肤牵拉闭合器联合RO-NPT联合治疗,其中,1例患者因合并重症肺炎死亡,35例患者创面疤痕质量评分为(3.0±1.0)分,褥疮愈合量表评分为(6.0±1.0)分,疼痛感在术后第3天逐渐减轻,最终消失,创面均愈合良好,症状消失,35例患者创面的有效率均高于70%,无愈合不良或不愈合情况;1例骶尾部褥疮患者因高龄,术前合并有较多基础疾病,术后因严重的心肺功能衰竭,肺部感染死亡。总的来说,19~50岁褥疮患者为(5.0±1.2)周,51~90岁褥疮患者为(7.0±2.1)周;糖尿病足患者的住院时间和术前患者血糖水平,合并基础疾病相关,其中,4例糖尿病足患者因血糖控制不佳,术后住院时间为(10±1.5)周,1例糖尿病足患者术前血糖控制良好,合并有较少基础疾病,术后住院时间为9 d;1例腹部巨大缺损患者术后住院时间为14 d,术后第28天拆除Top Closure皮肤牵拉闭合器,见表1。
表 1 36例患者创面疗效分析($\bar x \pm s$ )Table 1. Analysis of curative effect of 36 cases of wounds ($\bar x \pm s$ )疾病类型 人数(n) 年龄(岁) 疤痕质量评分(分) 褥疮愈合质量评分(分) 创面愈合情况 住院时间(周) 褥疮 5 19~50 2.0 ± 1.0 5.0 ± 2.0 痊愈 5.0 ± 1.2 25 51~90 3.0 ± 1.0 6.0 ± 1.0 24例痊愈
1例死亡7.0 ± 2.1 糖尿病足 5 65~80 3.0 ± 1.0 痊愈 10 ± 1.5 巨大缺损 1 35 3 痊愈 3 2.1 典型案例报告
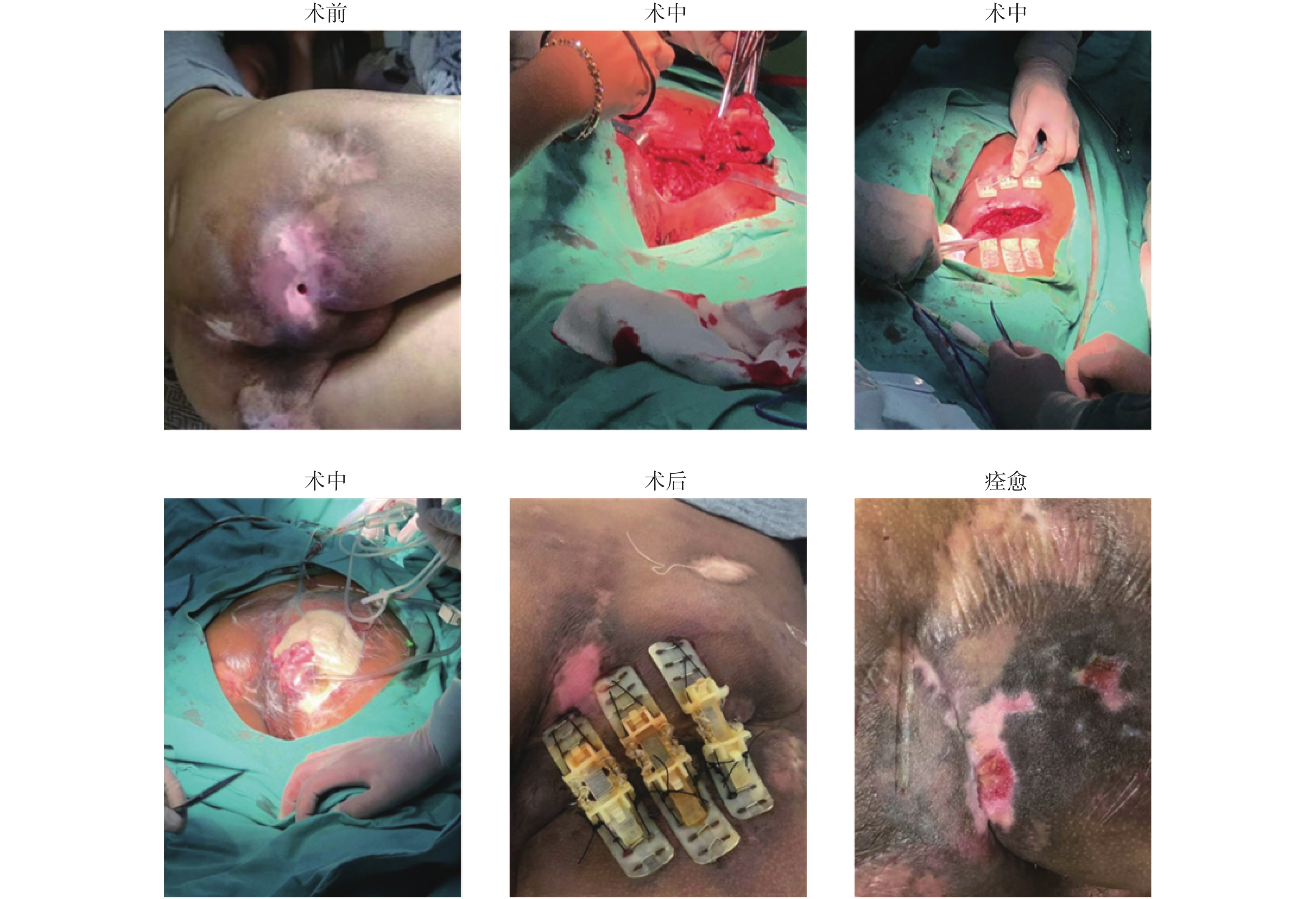
典型案例1:患者,男,48岁,因工地高处坠落致下肢截瘫,长期卧床,造成全身多发褥疮,其中臀部反复褥疮3 a,褥疮创面伴有脓性分泌物;同时因为褥疮创面临近肛门区域,粪便污染创面,造成反复感染,虽尝试运用多种新型创面敷料、清创引流技术均难以愈合,积极完善术前准备,全麻下行乙状结肠造口术 + Top Closure皮肤持续牵张术 + RO-NPT术,术中发现褥疮创面复杂且深及骨膜,故对患者病灶进行彻底切除,取出坏死组织,创面约8 cm×6 cm,术后患者安返病房,并持续给氧负压封闭引流治疗创面,术后持续治疗四周后对皮肤闭合牵张器进行拆除,继续对创面进行给氧+冲洗负压治疗修复,第6周后创面完全愈合,见图1。
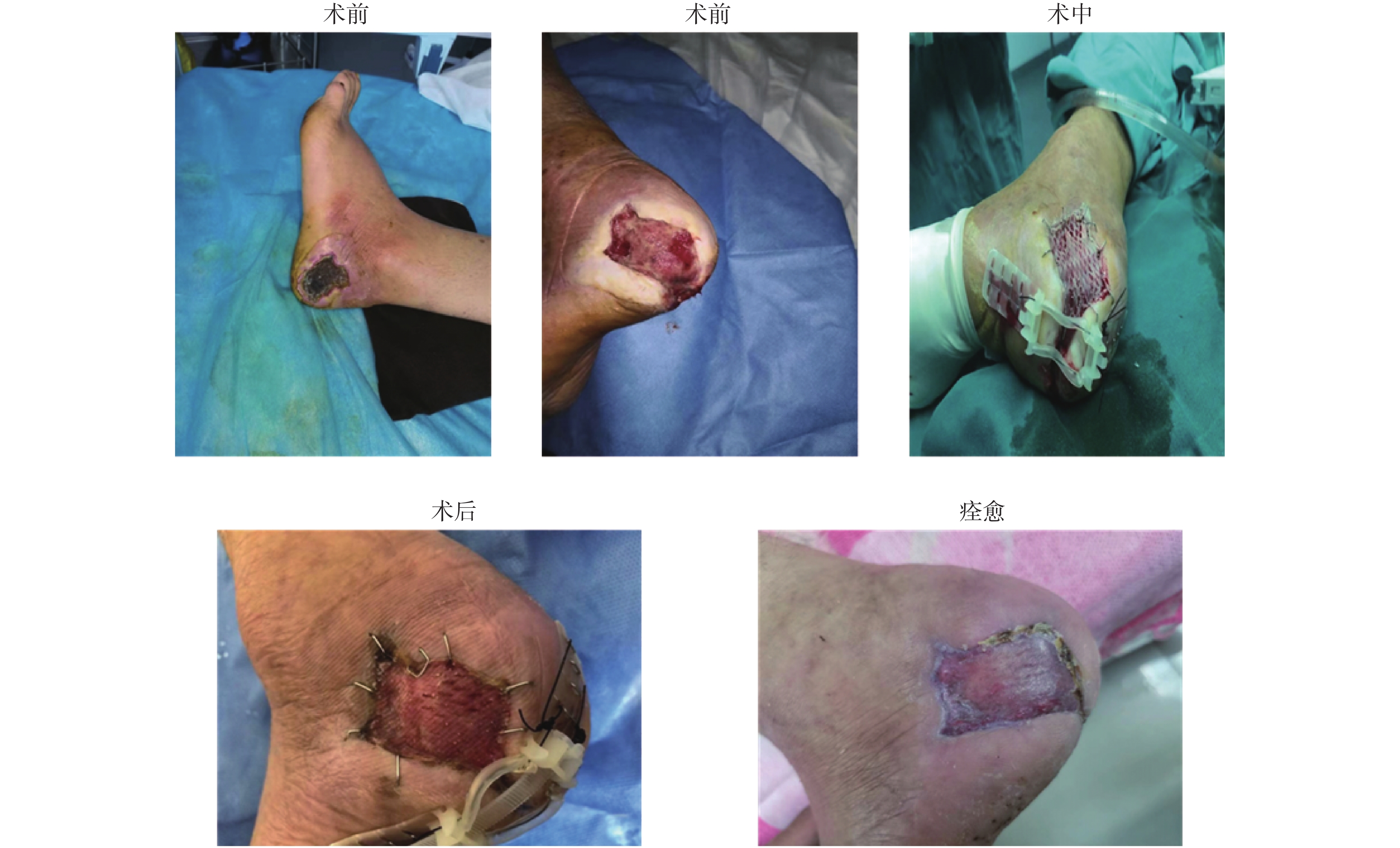
典型案例2:患者,男,56岁,患有糖尿病,未控制血糖,未定期监测血糖,入院前1月发现左足踝根部皮肤破溃,入院后腰麻下行左侧足部创面慢性溃疡清创修补术+自体皮片移植 + Top Closure皮肤持续牵张术 + RO-NPT术,术后患者安返病房,并持续给氧负压封闭引流治疗创面,术后持续治疗9 d后对皮肤闭合牵张器进行拆除,创面愈合,患者康复出院,见图2。
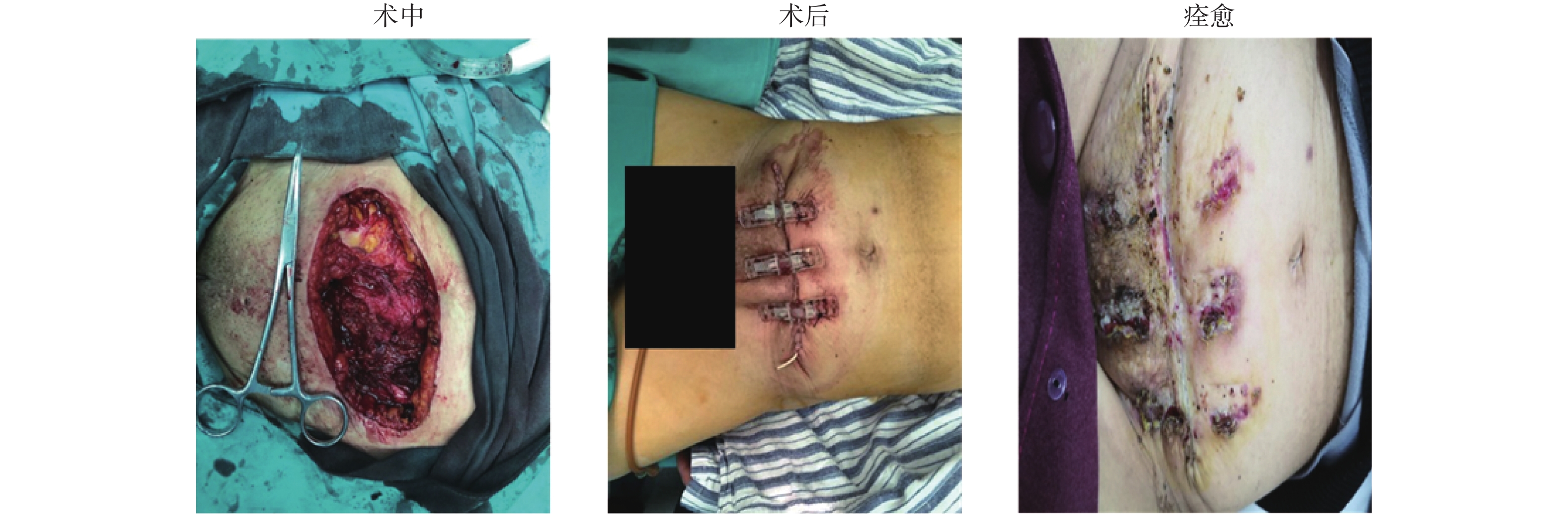
典型案例3:患者,女,25岁,因发现腹壁包块2 a入院,入院查体发现中下腹可触及大小约15 cm×20 cm的包块,质韧,活动性差,入院腹部CT(平扫 + 增强)检查结果提示:中下腹腹腔内,前腹壁肌层多发肿块,考虑恶性可能。积极完善术前准备,全麻下腹壁肿瘤切除术 + Top Closure皮肤持续牵张术,术后患者安返病房,术后病理检查结果提示:梭形细胞瘤。术后皮肤持续牵张技术持续治疗2周后患者出院,28 d之后患者再次入院拆除Top Closure皮肤牵拉闭合器,创面愈合良好,见图3。
2.2 治疗体会
褥疮患者往往为截瘫患者,长期卧床患者,常合并有糖尿病,心脑血管疾病等基础疾病,全身营养状况较差,时常合并有贫血,低白蛋白血症,治疗中应该注意控制血糖,对心肺功能等重要脏器功能的监测,应注意肠内肠外营养支持,纠正营养不良;老年患者手术麻醉的选择首选区域阻滞麻醉—连续硬膜外麻醉或局麻,尽量避免全麻插管后导致的难以控制的肺部感染;肛门区域的褥疮患者护理困难,笔者的经验是行肠道造瘘术使粪便转流以利于褥疮创面的清洁护理。糖尿病足患者治疗较为复杂,需制定包括全身支持治疗、患肢血管重建、清创坏死组织、创面修复以及康复锻炼、预防复发在内的综合保肢治疗方案。
3. 讨论
负压封闭引流是一种通过真空敷料促进急慢性伤口愈合的治疗技术,主要包括伤口敷料和引流管,以填充或覆盖皮肤或软组织缺损患者的伤口表面;生物半透膜将创面与敷料密封,形成封闭的微环境。当引流管与真空源连接时,会建立一个受控的负压,从伤口抽出液体,并增加流向受影响区域的血流量[12]。负压封闭引流是一种无创的辅助治疗方法,其耐受性好、禁忌症少和并发症少,可以减少水肿,能有效防止残余脓肿和死腔的形成,保护伤口免受污染,刺激创面和肉芽组织快速健康生长,促进创面愈合,减少抗生素的使用,进而有效促进动脉循环和营养供给,缩短住院时间,使伤口缩小,有效率高于常规治疗[13]。有循证医学证据[14]推荐负压封闭引流作为糖尿病足患者的首选治疗方法。RO-NPT在负压封闭引流的基础上补充氧气,不仅具有负压封闭引流的优点,还可以治疗患者对厌氧性伤口感染,预防和促进伤口愈合[15]。
皮肤持续牵张技术不破坏皮肤,可以实现一期皮肤闭合,无需植皮和皮瓣,减少了手术时间、住院时间和费用,改善了创面的美观,该技术能够将皮肤的粘弹性特性发挥到极致,通过应力松弛原理和机械蠕变机制扩大原发伤口闭合的限制[9]。另外,其手术相对简单,大大降低了手术难度,术后伤口愈合良好,可应用于大中创面的各种外科手术[8]。
胶原蛋白水解物又称胶原蛋白肽,是一种广泛应用的营养补充剂,是一种不同分子量肽的混合物,来自明胶,一种热变性的胶原蛋白,通过酶水解。口服摄入的胶原蛋白肽吸收为游离氨基酸和低聚肽,如脯氨酸羟脯氨酸和羟脯氨酸甘氨酸,研究表明,标准护理加上两种胶原蛋白水解物中的一种明显有助于压疮的愈合[16]。Iizaka S等[17]发现维持代谢平衡所需的能量需求(30 kcal/kg)和平均蛋白质需求(0.95 g/kg)的摄入与深部创面的伤口愈合相关,改善渗出液和坏死组织,但对于浅表性创面则没有相关性。对于难治性创面患者而言,营养支持显得尤为重要。
连续的褥疮创面评估是必要的,以检测褥疮恶化,愈合平台,或愈合的程度和质量。褥疮的疗效评估最广泛使用的是褥疮愈合量表(pressure ulcer scale for healing,PUSH)和褥疮状态工具(pressure sore status tool,PSST),其次是Sussman伤口愈合工具(the sussman wound healing tool,SWHT)和会话量表[18]。PUSH由3个参数组成:伤口表面积(0~10);渗出量(0~3);组织类型(0~4),3道题的分数可以相加得出总分(0~17),分数越低说明伤口情况越好[19]。PSST由13个伤口特征(大小、深度、边缘、凹陷、坏死组织类型、坏死组织数量、渗出类型、渗出量、伤口周围皮肤颜色、周围组织水肿、周围组织硬化、肉芽组织和上皮化)组成,并对这些特征进行评分和总结,得出创面的愈合情况。PSST的优点是它可以用来评估任何慢性伤口。然而,它比简单的PUSH工具需要更多的时间来完成[2]。
-
表 1 不同Wagner分级糖尿病足患者危险因素分析[n(%)/M(P25,P75)/$ \bar x \pm s $]
Table 1. Analysis of risk factors in diabetic foot patients with different Wagner grades [n(%)/M(P25,P75)/$ \bar x \pm s $]
项目 Wagner1、2级(n = 78) Wagner3级(n = 274) Wagner4级(n = 184) F/χ2 P 平均住院天数(d) 13(9.75,15.00) 15(13,18) 16(13.25,18) 25.662 < 0.010* 平均年龄(岁) 62.94 ± 11.456 60.85 ± 12.546 61.79 ± 11.805 0.991 0.372 平均糖尿病病程(a) 13(7,20) 10(6,20) 13(8,20) 3.725 0.155 糖尿病足病程(月) 1.00(0.23,2.00) 2.00(0.50,4.00) 2.00(0.66,4.00) 20.891 < 0.001* 白细胞计数(×109/L) 7.45(5.58,9.31) 8.87(7.09,10.92) 9.95(7.33,15.89) 37.479 < 0.001* 血沉(mm/L) 38.00(13.00,68.00) 68.00(39.75,80.00) 71.00(68.00,95.75) 56.681 < 0.001* 中性粒细胞计数(109/L) 68.15(57.00,74.12) 71.75(64.45,80.82) 77.7(68.17,86.62) 46.102 < 0.001* 白蛋白( g/L) 37.55 ± 4.62 34.77 ± 5.58 32.32 ± 6.16 43.897 < 0.001* 饮酒史 有 57(73.1) 178(65.0) 122(66.3) 1.808 0.405 无 21(26.9) 96(35.0) 62(33.7) 高血压 有 26(33.3) 119(43.4) 75(40.8) 2.568 0.277 无 52(66.7) 155(56.6) 109(59.2) 性别 男 50(64.1) 191(69.7) 120(65.2) 1.447 0.485 女 28(35.9) 83(30.3) 64(34.8) 吸烟史 有 57(73.1) 178(65.0) 122(66.3) 1.808 0.405 无 21(26.9) 96(35.0) 62(33.7) *P < 0.05。 表 2 主要革兰阳性球菌对常用抗菌药物的耐药率 [株(%)]
Table 2. Resistance rates of major Gram-positive cocci to common antimicrobial agents[strains(%)]
抗生素 金黄色葡萄
球菌(128株)粪肠球菌
(47株)表皮葡萄
球菌(21株)青霉素 115(89.84) 9(19.15) 7(33.33) 庆大霉素 20(15.62) 14(29.79) 1(4.76) 左旋氧氟沙星 33(25.78) 10(21.28) 4(19.05) 莫西沙星 22(17.19) 10((21.28) - 红霉素 79(61.72) 25(53.19) 5(23.81) 克林霉素 80(62.5) 31(65.96) 5(23.81) 复方新诺明 38(29.69) 1(2.13) 4(19.05) 苯唑西林 34(26.56) 5(10.64) 7(33.33) 四环素 43(33.59) 28(59.57) 6(28.57) 环丙沙星 22(17.19) 11(23.40) 2(9.52) 奎奴普丁 7(5.47) 25 1(4.76) 链霉素 - 4(8.51) - 利奈唑胺 - - 2(9.52) 阿莫西林 8(6.25) - 2(9.52) 氨苄西林 17(13.28) 3(6.38) 2(9.52) 达托霉素 1(0.78) - 1(4.76) 替加环素 6(4.69) - 3(14.29) 万古霉素 1(0.78) - 2(9.52) 表 3 主要革兰阴性球菌对常用抗菌药物的耐药率 [株(%)]
Table 3. Resistance rates of major Gram-negative cocci to common antimicrobial agents[strains(%)]
抗生素 大肠埃
希菌(57株)阴沟肠
杆菌(43株)肺炎克雷伯
杆菌(30株)阿莫西林 6(10.53) 33(76.74) 6(20.00) 头孢呋辛 18(31.58) 22(51.16) 12(40.00) 头孢西丁 7(12.28) 29(67.44) 3(10.00) 头孢他啶 5(8.77) 11(25.58) 6(20.00) 头孢曲松 15(26.32) 14(32,56) 7(23.30) 左旋氧氟沙星 18(31.58) 9(20.93) 9(30.00) 复方新诺明 28(49.12) 16(37.21) 18(60.00) 环丙沙星 8(14.03) 11(25.58) 8(26.67.00) 氨曲南 7(12.28) 11(25.58) 6(20.00) 氨苄西林 45(78.94) 33(76.74) 22(73.30) 头孢唑林 28(49.12) 30(69.77) 16(53.30) 亚胺培南 2(3.50) 4(9.30) 3(10.00) 庆大霉素 17(29.82) 9(20.93) 5(16.67) 环丙沙星 5(8.77) - - 哌拉西林 14(24.56) 17(39.54) 7(23.33) 头孢吡肟 4(7.01) 3(6.98) 5(16.67) 呋喃妥因 4(7.01) 2(4.65) 7(23.33) 妥布霉素 1(1.75) 6(13.95) 3(10.00) 阿卡米星 - - 3(10.00) 表 4 不同Wagner分级与不同病原菌的差异性分析[n(%)]
Table 4. Differential analysis of different wagner grades and different pathogens [n(%)]
病原菌分类 Wagner1、2级(n = 78) Wagner3级(n = 274) Wagner4、5级(n = 184) F/χ2 P 革兰阳性菌 51(65.4) 144(52.6) 71(39.7) 19.227 0.004* 革兰阴性菌 21(26.9) 103(37.6) 90(48.9) 真菌 1(1.3) 26(9.5) 21(11.4) 多种细菌混合 5(6.4) 26(9.5) 21(11.4) *P < 0.05。 -
[1] Wukich D K,Crim B E,Frykberg R G,et al. Neuropathy and poorly controlled diabetes increase the rate of surgical site infection after foot and ankle surgery[J]. Journal of Bone and Joint Surgery,2014,96(10):832-839. doi: 10.2106/JBJS.L.01302 [2] 谷涌泉,冉兴无,郭连瑞,等. 中国糖尿病足诊治指南[J]. 中国临床医生杂志,2024,52(11):1287-1296. doi: 10.3969/j.issn.2095-8552.2024.11.007 [3] 蒋竹奕,吴炎,谢颖,等. 糖尿病足感染181例临床分析[J]. 中国感染与化疗杂志,2021,21(5):517-522. [4] 牛文芳,朱平,史琳涛,等. 糖尿病足感染病原菌与Wagner分级相关性研究[J]. 世界临床药物,2019,40(10):737-741. [5] Macdonald K E,Boeckh S,Stacey H J,et al. The microbiology of diabetic foot infections: A meta-analysis[J]. BMC Infectious Diseases,2021,21(1):770. doi: 10.1186/s12879-021-06516-7 [6] Kulas J A,Weigel T K,Ferris H A. Insulin resistance and impaired lipid metabolism as a potential link between diabetes and Alzheimer’ s disease. [J]. Drug Development Research,2020,81(2):194-205. [7] Macdonald K E,Boeckh S,Stacey H J,et al. The microbiology of diabetic foot infections: A meta-analysis[J]. BMC Infectious Diseases,2021,21(1):770. doi: 10.1186/s12879-021-06516-7 [8] Turzańska K,Adesanya O,Rajagopal A,et al. Improving the management and treatment of diabetic foot infection: Challenges and research opportunities[J]. International Journal of Molecular Sciences,2023,24(4):3913. doi: 10.3390/ijms24043913 [9] 中华医学会糖尿病学分会,中华医学会感染病学分会,中华医学会组织修复与再生分会. 中国糖尿病足防治指南(2019版)(Ⅰ)[J]. 中华糖尿病杂志,2019,11(2):92-108. doi: 10.3760/cma.j.issn.1674-5809.2019.02.004 [10] 张会峰,许樟荣,冉兴无. 糖尿病足的相关定义和标准[J]. 中华糖尿病杂志,2020,12(6):363-368. doi: 10.3760/cma.j.cn115791-20200430-00258 [11] Clinical and Laboratory Standards Institute. M100S. Performance standards for antimicrobial susceptibility testing:Twenty-sixth edition[S].Wayne, PA:CLSI,2016. [12] Everett E, Mathioudakis N. Update on management of diabetic foot ulcers[J]. Annals of the New York Academy of Sciences,2018,1411(1):153-165. [13] Armstrong D G, Tan T W, Boulton A J M, et al. Diabetic foot ulcers[J]. JAMA,2023,330(1):62-75. [14] 李玲艳, 赵寒, 李佳璐, 等. 糖尿病足综合管理研究进展[J]. 中国护理管理,2022, 22(8):1271-1275. [15] Senneville É, Albalawi Z, van Asten S A, et al. IWGDF/IDSA guidelines on the diagnosis and treatment of diabetes‐related foot infections (IWGDF/IDSA 2023)[J]. Diabetes/Metabolism Research and Reviews,2024,40(3):e3687. [16] 张红妹, 陈育群. 糖尿病足患者延误就医原因的质性研究[J]. 护理学报,2011, 18(2B4):25-27. [17] 陈玉凤, 李江雁, 毛小芳, 等. 糖尿病足感染病原菌分布及临床特征分析[J]. 中国病原生物学杂志,2022,17(8):942-946. [18] 麦惠盈, 张德昊, 潘南芳, 等. 海南地区糖尿病足坏疽的风险因素分析[J]. 中国热带医学,2024,24(5):584-590. [19] Du F, Ma J, Gong H, et al. Microbial infection and antibiotic susceptibility of diabetic foot ulcer in China: Literature review[J]. Frontiers in Endocrinology,2022,19(13):881659-881659. doi: 10.1111/nyas.13569 [20] 刘肖肖, 刘莉, 董海丽, 等. 糖尿病足病人截肢决定因素的研究进展[J]. 全科护理,2024,22(15):2838-2842. doi: 10.1001/jama.2023.10578 [21] 李梦文, 刘晓梅, 孙宁, 等. 住院糖尿病患者高危足部风险及影响因素研究[J]. 护理管理杂志,2024,24(10):882-886. [22] 张加其, 姜晓锐, 王凯, 等. 糖尿病足感染患者的病原菌类型与病例特点及预后的相关性[J]. 医药导报,2022,41(9):1360-1365. [23] 严春艳, 袁映梅, 郭爱梅, 等. 中国糖尿病患者就医延迟研究现状文献分析[J]. 老年医学研究,2023,4(5):38-41. [24] 彭倩, 吴英, 宋佳雪, 等. 糖尿病足患者就医延迟原因质性研究的Meta整合[J]. 军事护理,2024,41(6):90-93. [25] Zhang P, Lu J, Jing Y, et al. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis[J]. Annals of Medicine,2017,49(2):106-116. [26] Lim J Z, Ng N S, Thomas C. Prevention and treatment of diabetic foot ulcers[J]. Journal of the Royal Society of Medicine,2017,110(3):104-109. -









 下载:
下载:

 下载:
下载:


