Efficacy Analysis of Combined Prediction of Postpartum Hypertension by 24-hour Urinary Protein,Hematocrit-albumin,and BNP in Patients with Severe Preeclampsia at the End of Pregnancy
-
摘要:
目的 探讨重度子痫前期患者妊娠末期24 h尿蛋白定量(24-hUP)、红细胞压积与血浆白蛋白差值(HCT-ALB)、脑钠肽(BNP)联合预测产后高血压的效能。 方法 采用整群抽样法,回顾性选取重庆医科大学附属大学城医院2018年1月—2022年12月540例重度子痫前期患者,根据是否发生产后高血压分为高血压组(n = 98)与非高血压组(n = 442)。比较两组临床资料[年龄、体质量指数(BMI)、产妇类型、流产史、高血压家族史、吸烟史、总胆固醇(TC)、甘油三酯(TG)、空腹血糖(FBG)、收缩压(SBP)、舒张压(DBP)]及妊娠末期24-hUP、HCT-ALB、BNP水平,分析妊娠末期24-hUP、HCT-ALB、BNP水平对产后高血压的预测价值。 结果 高血压组BMI、高血压家族史、TC、TG、FBG、SBP、DBP水平高于非高血压组[(25.63±1.37) kg/m2 vs (23.05±1.23) kg/m2,70.41% vs 30.54%,(5.32±1.14) mmol/L vs (3.91±0.95) mmol/L,(3.48±0.82) mmol/L vs (1.66±0.43) mmol/L,(7.24±1.60) mmol/L vs (4.83±1.22) mmol/L,(148.27±13.29) mmHg vs (127.65±10.71) mmHg,(92.36±5.17) mmHg vs (84.20±4.35) mmHg],差异有统计学意义(P < 0.05);高血压组妊娠末期24-hUP、HCT、HCT-ALB、BNP水平高于非高血压组[(7.82±2.18) g/24 h vs (6.15±1.26) g/24 h,(34.22±3.15)% vs (32.80±1.77)%,(6.19±2.01) vs (3.46±0.90),(646.43±170.59) pg/mL vs (523.81±134.62) pg/mL],ALB水平低于非高血压组[(28.03±1.13) g/L vs (29.34±1.44) g/L],差异有统计学意义(P < 0.05);妊娠末期24-hUP、HCT、HCT-ALB、BNP与SBP、DBP呈正相关,ALB与之呈负相关,差异有统计学意义(P < 0.05);妊娠末期24-hUP、HCT-ALB、BNP是产后高血压的独立危险因素,各指标联合预测产后高血压的AUC为0.930(95%CI:0.905~0.950),约登指数为0.719,敏感度为85.71%,特异度为86.20%,且联合预测的AUC明显大于三者单独预测,差异有统计学意义(P < 0.05)。 结论 妊娠末期24-hUP、HCT-ALB、BNP是产后高血压的独立危险因素,联合预测效能明显优于单一指标,可作为临床预测重度子痫前期患者是否发生产后高血压的优选方式。 Abstract:Objective To investigate the efficacy of combined prediction of postpartum hypertension using 24-h urinary protein quantification (24-hUP), hematocrit and plasma albumin difference (HCT-ALB), and brain natriuretic peptide (BNP) in patients with severe preeclampsia at the end of pregnancy. Methods A retrospective study was conducted using cluster sampling to select 540 patients with severe preeclampsia from the University City Hospital affiliated to Chongqing Medical University between January 2018 and December 2022 . Patients were divided into a hypertension group (n = 98) and a non-hypertension group (n = 442) based on the occurrence of postpartum hypertension. Clinical data [age, body mass index (BMI), maternal type, abortion history, family history of hypertension, smoking history, total cholesterol (TC), triglyceride (TG), fasting blood glucose (FBG), systolic blood pressure (SBP), diastolic blood pressure (DBP)] and levels of 24-hour urinary protein excretion (UP), hematocrit-albumin (HCT-ALB), and BNP in the third trimester of pregnancy were compared between the two groups to analyze the predictive value of these indicators for postpartum hypertension. Results The levels of BMI, family history of hypertension, TC, TG, FBG, SBP and DBP in hypertensive group were higher than those in non-hypertensive group [(25.63±1.37) kg/m2 vs (23.05±1.23) kg/m2, 70.41% vs 30.54%]. (5.32±1.14) mmol/L vs (3.91±0.95) mmol/L, (3.48±0.82) mmol/L vs (1.66±0.43) mmol/L, (7.24±1.60) mmol/L vs (4.83±1.22) mmol/L, (148.27±13.29) mmHg vs (127.65±10.71) mmHg, (92.36±5.17) mmHg vs (84.20±4.35) mmHg], the difference was statistically significant (P < 0.05). The levels of urinary protein, HCT, HCT-ALB and BNP at 24 h at the end of pregnancy in hypertension group were also higher than those in non-hypertension group [(7.82±2.18) g/24 h vs (6.15±1.26) g/24 h, (34.22±3.15) % vs (32.80±1.77) %]. (6.19±2.01) vs (3.46±0.90), (646.43±170.59) pg/mL vs (523.81±134.62) pg/mL], while ALB level was lower than that of the non-hypertension group [(28.03±1.13) g/L vs (29.34±1.44) g/L], with statistically significant differences (P < 0.05). There was a positive correlation between 24-hUP, HCT, HCT-ALB, BNP and SBP, DBP, while ALB was negatively correlated with SBP and DBP, the difference was statistically significant(P < 0.05). 24-hUP, HCT-ALB and BNP at the end of pregnancy were independent risk factors for postpartum hypertension, with a combined prediction AUC of 0.930 (95%CI: 0.905~0.950), a Jordon index of 0.719, sensitivity of 85.71%, the specificity of 86.20%. The AUC of the combined prediction was significantly greater than that of each individual predictor, with statistically significant differences(P < 0.05). Conclusion 24-hUP, HCT-ALB, and BNP at the end of pregnancy are independent risk factors for postpartum hypertension. Their combined predictive efficacy is significantly superior to that of individual indicators and can be used as an optimal clinical method for predicting whether patients with severe preeclampsia will develop postpartum hypertension. -
糖尿病(diabetes mellitus,DM)是一组以慢性高血糖为特征的代谢性疾病。糖尿病周围神经病变(diabetic peripheral neuropathy,DPN)是糖尿病最常见的并发症之一,国内患病率达67.6%,其中重度 DPN患病率达19.3%[1]。雪旺细胞作为周围神经系统中最重要的髓鞘细胞,参与维持周围神经的正常结构和功能[2-3],炎症反应对雪旺细胞的损伤是DPN发生发展的重要因素[4]。核因子κB(nuclear factor kappa-b,NF-κb)级联反应是炎症反应的调控中心,抑制NF-κb通路,有望降低炎性细胞因子的表达,最终改善神经损伤[5]。
Mer受体酪氨酸激酶(Mer receptertyroshin kinase,Mertk)是受体酪氨酸激酶家族TAM成员之一,作为一种单跨膜受体可以通过结合多种配体将信号从细胞外基质转导至细胞质,调节众多生理过程[6-8]。现有文献显示[9-10],Mertk能够抑制促炎性细胞因子IL-12、干扰素和NF-κb的表达,从而抑制炎症信号转导。本课题前期利用公共数据库,发现并报道[11]Mertk基因表现出糖尿病周围神经病变的疾病特异性,但Mertk在雪旺细胞中的可能作用及其调控机制尚不清楚。为此,本研究通过沉默大鼠雪旺细胞内Mertk基因,探究Mertk是否通过调控炎症反应NF-κb通路相关蛋白B细胞κ轻肽基因增强子抑制因子激酶ε(inhibitor kappa B kinaseβ,Ikbkb)、P65、TNF-α,从而影响雪旺细胞炎症反应。
1. 材料与方法
1.1 主要仪器与试剂
AI1600超灵敏化学发光成像仪(GE公司,美国)和正置荧光显微镜(Olympus公司,日本)来源于昆明医科大学科技成果孵化中心,垂直电泳及湿式转膜系统购自北京六一生物科技公司。葡萄糖浓度为4.5 g/L(25 mmol/L)的DMEM培养基、胎牛血清、青霉素-链霉素双抗和含EDTA的0.25%胰蛋白酶消化液均购自美国GIBCO公司,无水葡萄糖购自广州光华科技有限公司,BCA蛋白浓度测定试剂盒购自上海碧云天公司,Mertk、Ikbkb抗体购自美国Abcam公司,P65抗体、TNF-α抗体、β-actin抗体、IgG抗体、抗兔/抗鼠第二抗体购自武汉Proteintech公司,免疫荧光试剂盒购自北京索莱宝科技有限公司,免疫共沉淀试剂盒购自上海爱必信科技有限公司。
1.2 细胞培养
大鼠雪旺氏细胞株(RSC96)来自昆明医科大学科技成果孵化中心,生长所需细胞培养基配比是DMEM培养基∶胎牛血清∶双抗=100∶10∶1,放置在37℃、5% CO2饱和湿度的细胞培养箱中静置培养。
1.3 Mertk在高糖环境雪旺细胞中表达
将RSC96细胞随机分为对照组(25 mmol/L组)和处理组(50、75、100及125 mmol/L组)。首先使用不添加胎牛血清的培养基进行饥饿处理4 h,再将DMEM培养基中葡萄糖浓度分别调整至50 mmol/L、75 mmol/L、100 mmol/L及125 mmol/L,继续培养48 h,Western blot检测细胞内Mertk蛋白表达水平。
1.4 免疫共沉淀检测内源性Mertk、Ikbkb相互作用
培养3瓶RSC96细胞。以Lysis buffer∶PMSF=100∶1配制裂解液,将RSC96细胞裂解、超声、离心、留取Input蛋白,所得产物分别加入Mertk、Ikbkb、IgG一抗1~5 µg,4℃孵育过夜后加入5 µL Protein A与Protein G,再经过孵育、洗涤、离心、SDS缓冲液重悬、变性得到终产物,最后利用免疫印迹法检测Mertk与Ikbkb的相互作用。
1.5 免疫印迹法检测沉默Mertk后RSC96内Ikbkb、P65、TNF-α表达水平
将3种Mertk siRNA试剂(siRNA1 /siRNA2 /siRNA3)及空载对照(NC)利用GP-Transfect-Mate试剂转染进入RSC96细胞。48 h后根据BCA试剂盒说明书所述提取总蛋白,免疫印迹法检测Mertk、Ikbkb、P65、TNF-α表达水平。siRNA核苷酸序列见表1。
表 1 Mertk小干扰核苷酸序列Table 1. Mertk-RNA oligo sequences寡核苷酸 正义序列(5′-3′) 反义序列(5′-3′) Mertk-Rat-1 GGGUCGUACAUCUGUAAGATT UCUUACAGAUGUACGACCCTT Mertk-Rat-2 CACCCACUGAAGUCCAUAUTT AUAUGGACUUCAGUGGGUGTT Mertk-Rat-3 GAGGAAAGAUACAUCUUAATT UUAAGAUGUAUCUUUCCUCTT 1.6 免疫荧光法检测沉默Mertk后炎症因子TNF-α表达
在玻璃爬片上培养RSC96细胞,随机分为对照组(Con组)和实验组(siRNA3组),按组别处理细胞。48 h后细胞经过固定、通透、封闭等步骤后,分别加入Mertk(1∶200)、Ikbkb(1∶200)、P65(1∶200)一抗4℃孵育过夜,第2天加入相对应种属的荧光二抗暗室内孵育;DAPI染细胞核10 min后,将细胞爬片倒扣在滴有荧光淬灭剂的载玻片上,使用正置荧光显微镜观察并拍照。
1.7 统计学处理
采用SPSS23.0统计软件。计量资料采用Kolmogorov-Smirnov法检验数据正态性,符合正态分布时以均数±标准差($\bar x \pm s $)表示,多组间单因素比较采用单因素方差分析。P < 0.05为差异有统计学意义。
2. 结果
2.1 Mertk在雪旺细胞的表达
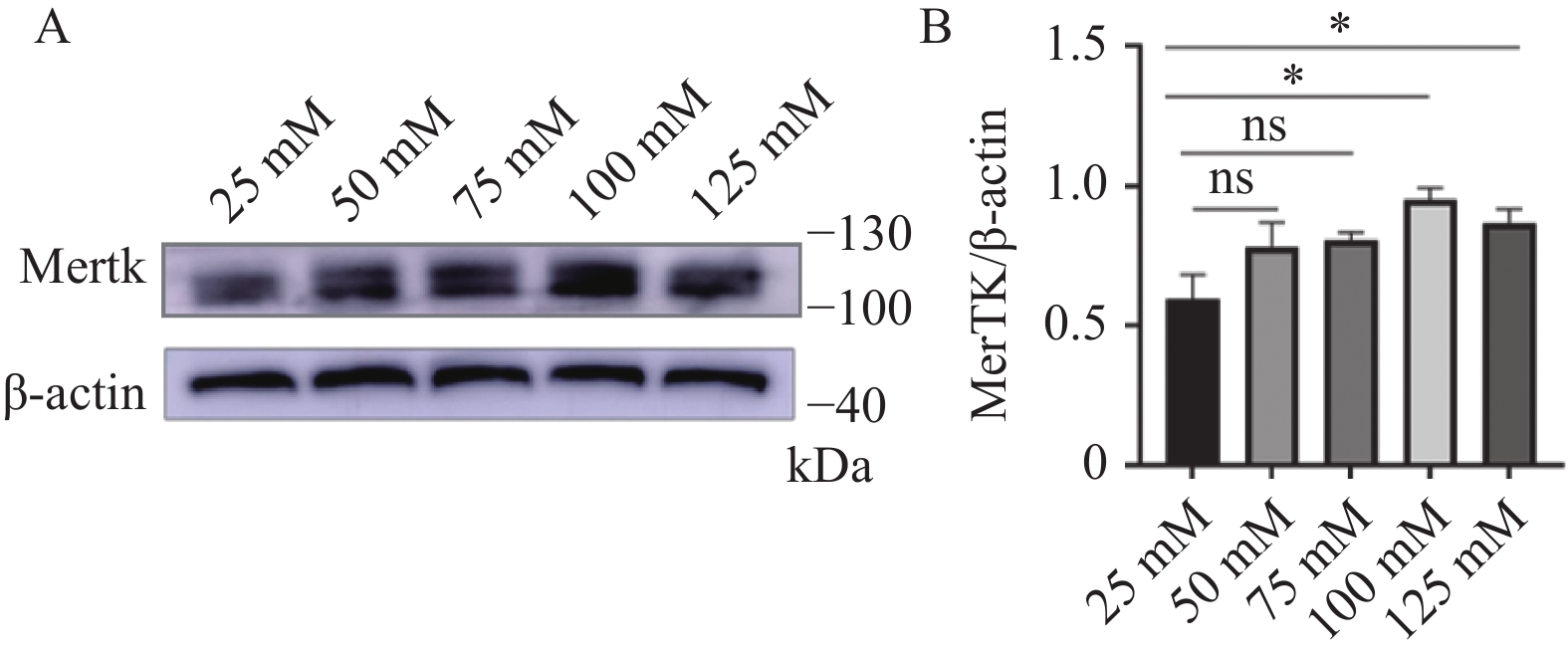
首先明确Mertk在雪旺细胞中是否表达,以及模拟糖尿病高糖环境下Mertk表达水平的变化情况。免疫印迹结果显示,Mertk在雪旺细胞中存在表达,且随葡萄糖浓度增加,细胞Mertk表达水平增加(P < 0.05),见表2和图1。
表 2 不同葡萄糖水平RSC96内Mertk蛋白表达Table 2. Mertk protein expression within RSC96 at different glucose levels灰度值
比值组别 F P 25 mmol/L 50 mmol/L 75 mmol/L 100 mmol/L 125 mmol/L Mertk/β-actin 0.60 ± 0.20 0.78 ± 0.19 0.81 ± 0.06 0.95 ± 0.09* 0.87 ± 0.11* 4.452 0.0098* 与对照组(25 mmol/L组)比较,*P < 0.05。 2.2 Mertk与Ikbkb内源性相互作用
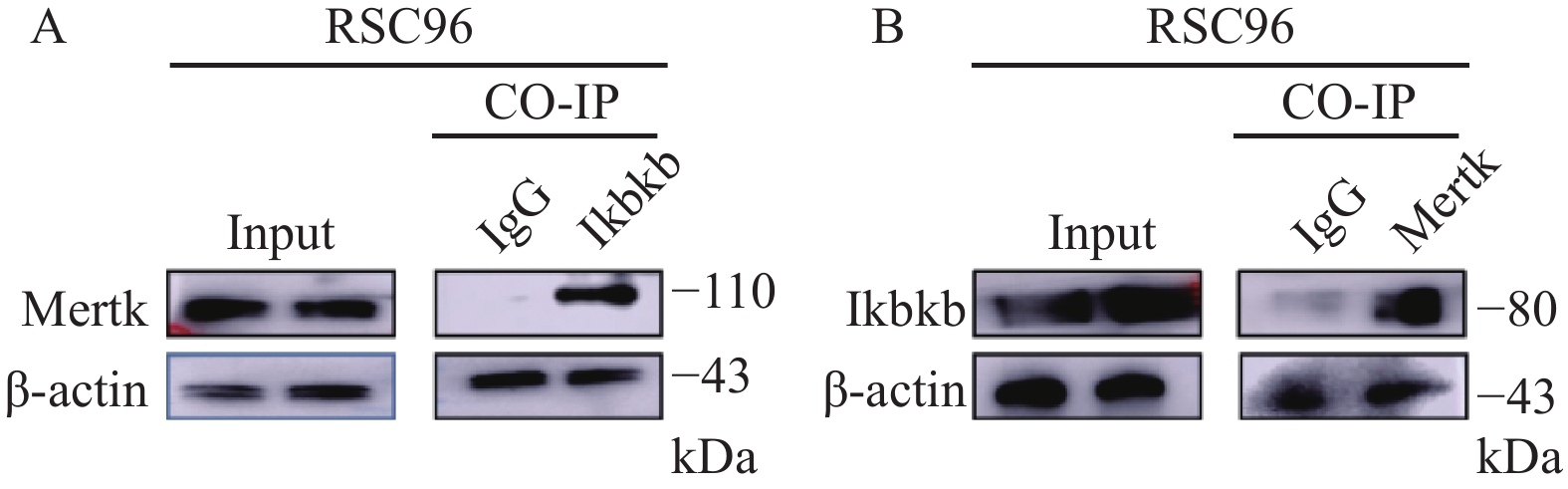
以非特异免疫的同源抗体IgG作为阴性对照,以Input作为阳性对照,免疫共沉淀结果显示,RSC96细胞内同时表达Mertk、Ikbkb,且二者之间存在相互作用关系,见图2。
2.3 沉默Mertk对雪旺细胞Ikbkb、P65、TNF-α蛋白表达的影响
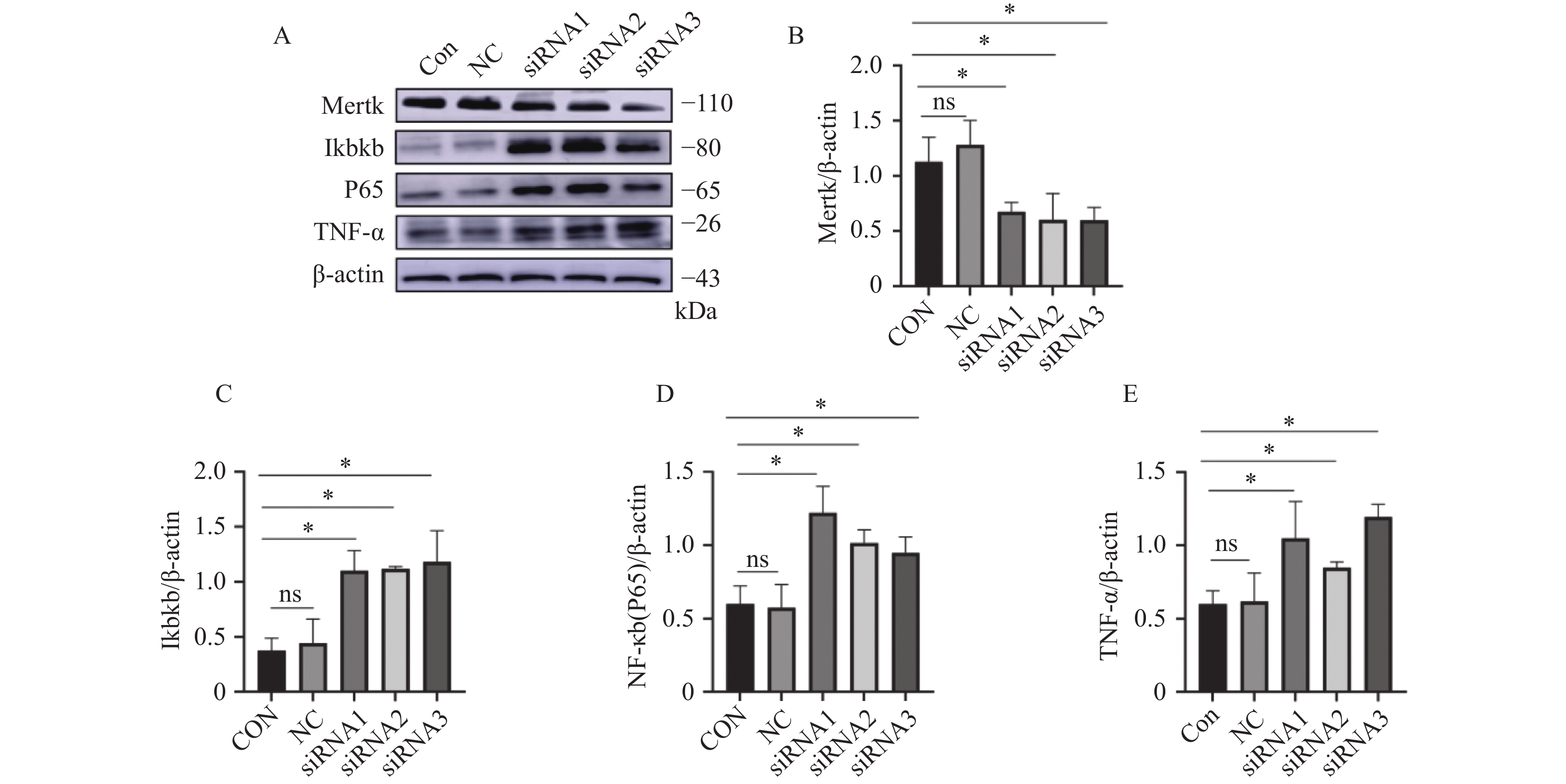
免疫印迹实验结果显示,3种siRNA均使Mertk表达含量下降(P < 0.05),同时Ikbkb、P65、TNF-α表达水平随Mertk表达水平的降低而升高(P < 0.05),见表3和图3。
表 3 沉默Mertk后Ikbkb、P65、TNF-α蛋白表达量Table 3. Ikbkb,P65,and TNF-α protein expression after silencing Mertk组别 Mertk
/β-actinIkbkb
/β-actinP65
/β-actinTNF-α
/β-actinCON 1.13 ± 0.22 0.38 ± 0.11 0.60 ± 0.12 0.60 ± 0.09 NC 1.28 ± 022 0.44 ± 0.22 0.57 ± 0.16 0.62 ± 0.19 siRNA1 0.68 ± 0.08* 1.10 ± 0.18* 1.22 ± 0.18* 1.05 ± 0.25* siRNA2 0.60 ± 0.24* 1.12 ± 0.02* 1.02 ± 0.09* 0.85 ± 0.04* siRNA3 0.60 ± 0.12* 1.18 ± 0.28* 0.95 ± 0.11* 1.19 ± 0.09* F 8.927 13.76 12.62 8.646 P 0.0025* 0.0004* 0.0006* 0.0028* 与CON组比较,*P < 0.05。 2.4 免疫荧光检测Mertk、Ikbkb、P65蛋白
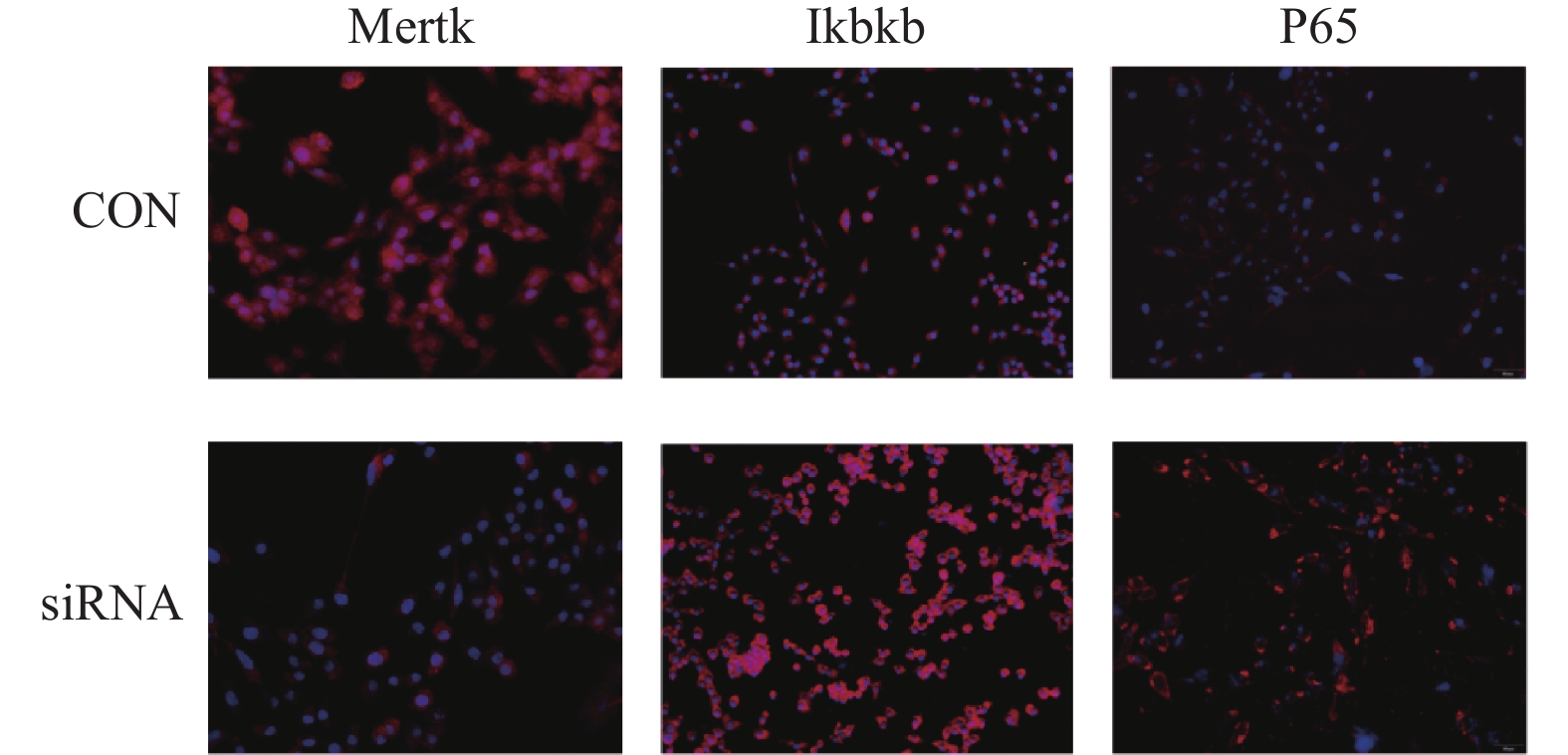
以siRNA3转染RSC96细胞后,Mertk、Ikbkb、P65蛋白免疫荧光结果与免疫印迹实验一致,见图4。
3. 讨论
糖尿病周围神经病是糖尿病患者最常见的慢性并发症,是导致糖尿病患者截肢,生活质量下降及死亡的主要原因之一,加重社会负担[12]。糖尿病周围神经病变以神经纤维脱髓鞘、神经传导速度受损为特征[13],而雪旺细胞正是周围神经系统中最重要的髓鞘细胞;雪旺细胞损伤与凋亡是糖尿病周围神经病变的重要病理学特征之一,也是导致糖尿病髓鞘功能不良的主要因素[14]。因此,本研究以大鼠雪旺细胞作为研究对象,以此作为糖尿病周围神经病变研究的重要切入点,并且首先明确了Mertk在雪旺细胞中的表达,即随着细胞生长环境内葡萄糖浓度的提高,Mertk表达量也随之提高。
蛋白质通常不是作为单一物质发挥作用,而是以蛋白质-蛋白质相互作用的方式在活细胞生物过程中发挥作用。本研究结果通过免疫共沉淀实验,验证了雪旺细胞内Mertk与Ikbkb的内源性相互作用。Ikbkb(也称IKKβ、IKK2)是IKK复合体的催化亚基之一,而IKK复合体是NF-κb信号转导通路的关键调节因子,可迅速激活NF-κb,以协调大多数靶基因的表达[15-16]。因此,本实验试图进一步探究Mertk是否间接调控NF-κb通路,对雪旺细胞炎症反应产生影响。
为达到这一目的,本研究使用siRNA敲减沉默表达Mertk后,发现P65及其下游炎症因子TNF-α表达水平升高,表明NF-κb通路受到正向调控,雪旺细胞内炎症反应加重。经典的NF-κb系统是由亚基p50和P65组成的复合体,在大多数静息细胞中,NF-κb以抑制物IκB的形式存在。糖尿病病理过程产生的刺激,可导致IKK特异性地磷酸化IκB,进而允许p50和P65向细胞核发出信号,激活大量有关基因[17]。NF-κb通路调控的促炎细胞因子是神经血管损伤和神经传导速度受损的主要原因,抑制NF-κb通路,有望降低炎性细胞因子的表达,最终改善神经损伤[5]。由此,课题组推测,以Mertk基因为靶点进行研究可能为糖尿病周围神经病的治疗提供新的思路。
综上所述,本研究以雪旺细胞为研究对象,发现Mertk与Ikbkb相互作用,沉默Mertk可上调Ikbkb,继而激活NF-κb信号转导通路,上调P65及其下游炎症因子TNF-α表达水平。本研究对糖尿病周围神经病变的发病机制、临床药物的治疗、靶向药物的研发都有非常积极的作用,这些体外细胞实验也为进一步的动物体内实验以及临床血清分子标记验证打下了基础。
-
表 1 两组临床资料比较[($ \bar x \pm s $)/n(%)]
Table 1. Comparison of clinical data between two groups [($ \bar x \pm s $)/n(%)]
项目 高血压组
(n = 98)非高血压组
(n = 442)t/χ2 P 年龄(岁) 28.76 ± 2.31 28.41 ± 2.07 1.482 0.139 BMI(kg/m2) 25.63 ± 1.37 23.05 ± 1.23 产妇类型 0.026 0.872 初产妇 55(56.12) 252(57.01) 经产妇 43(43.88) 190(42.99) 流产史 0.063 0.802 无 74(75.51) 339(76.70) 有 24(24.49) 103(23.30) 吸烟史 0.119 0.730 无 67(68.37) 310(70.14) 有 31(31.63) 132(29.86) 高血压家族史 54.233 < 0.001* 无 29(29.59) 307(69.46) 有 69(70.41) 135(30.54) TC(mmol/L) 5.32 ± 1.14 3.91 ± 0.95 12.795 < 0.001* TG(mmol/L) 3.48 ± 0.82 1.66 ± 0.43 31.209 < 0.001* FBG(mmol/L) 7.24 ± 1.60 4.83 ± 1.22 16.645 < 0.001* SBP(mmHg) 148.27 ± 13.29 127.65 ± 10.71 16.461 < 0.001* DBP(mmHg) 92.36 ± 5.17 84.20 ± 4.35 16.209 < 0.001* *P < 0.05。 表 2 两组妊娠末期24-hUP、HCT-ALB、BNP水平比较($\bar x \pm s $)
Table 2. Comparison of 24-hUP,HCT-ALB,and BNP levels between two groups during late pregnancy ($ \bar x \pm s $)
组别 n 24-hUP(g/24 h) HCT(%) ALB(g/L) HCT-ALB BNP(pg/mL) 高血压组 98 7.82 ± 2.18 34.22 ± 3.15 28.03 ± 1.13 6.19 ± 2.01 646.43 ± 170.59 非高血压组 442 6.15 ± 1.26 32.80 ± 1.77 29.34 ± 1.44 3.46 ± 0.90 523.81 ± 134.62 t 10.181 6.093 8.445 20.721 7.746 P < 0.001* < 0.001* < 0.001* < 0.001* < 0.001* *P < 0.05。 表 3 妊娠末期24-hUP、HCT-ALB、BNP与SBP、DBP的相关性
Table 3. Correlation between 24-hUP,HCT-ALB,BNP and SBP,DBP in late pregnancy
指标 SBP DBP r P r P 24-hUP 0.683 < 0.001* 0.691 0.042* HCT 0.511 0.034* 0.507 0.003* ALB −0.489 0.019* −0.493 < 0.001* HCT-ALB 0.626 < 0.001* 0.644 0.004* BNP 0.649 < 0.001* 0.672 < 0.001* *P < 0.05。 表 4 妊娠末期24-hUP、HCT-ALB、BNP对产后高血压的影响
Table 4. Effects of 24-hUP,HCT-ALB,and BNP in late pregnancy on postpartum hypertension
变量 β S.E. Waldχ2 OR 95%CI P 24-hUP 2.106 0.441 22.814 8.218 2.247~30.059 < 0.001* HCT-ALB 1.942 0.528 13.530 6.974 1.753~27.741 < 0.001* BNP 1.921 0.603 10.151 6.829 2.034~22.928 < 0.001* *P < 0.05。 表 5 ROC曲线对比结果
Table 5. Comparison results of ROC curve
成对对比 AUC差异 标准误差 95%CI Z P 联合-24-hUP 0.149 0.028 0.094~0.205 5.248 < 0.001* 联合-HCT-ALB 0.117 0.026 0.066~0.167 4.486 < 0.001* 联合-BNP 0.147 0.032 0.084~0.209 4.616 < 0.001* *P < 0.05。 -
[1] Yang Y Y,Le Ray I,Zhu J,et al. Preeclampsia prevalence,risk factors,and pregnancy outcomes in Sweden and China[J]. JAMA Netw Open,2021,4(5):e218401-e218401. doi: 10.1001/jamanetworkopen.2021.8401 [2] Chang K J,Seow K M,Chen K H. Preeclampsia: Recent advances in predicting,preventing,and managing the maternal and fetal life-threatening condition[J]. Int J Environ Res Public Health,2023,20(4):2994-3006. doi: 10.3390/ijerph20042994 [3] Muteke K,Musaba M W,Mukunya D,et al. Postpartum resolution of hypertension,proteinuria and acute kidney injury among women with preeclampsia and severe features at Mulago National Referral Hospital,Uganda: A cohort study[J]. Afr Health Sci,2023,23(3):27-36. [4] Stamilio D M,Beckham A J,Boggess K A,et al. Risk factors for postpartum readmission for preeclampsia or hypertension before delivery discharge among low-risk women: A case-control study[J]. Am J Obstet Gynecol MFM,2021,3(3):100317. doi: 10.1016/j.ajogmf.2021.100317 [5] Herman H G,Barda G,Miremberg H,et al. Management of pregnancies with suspected preeclampsia based on 6-hour vs 24-hour urine protein collection-a randomized double-blind controlled pilot trial[J]. Am J Obstet Gynecol MFM,2021,3(5):100429. doi: 10.1016/j.ajogmf.2021.100429 [6] 程敏,李青,李志芳. 红细胞压积及白蛋白指标评估子痫前期治疗效果的应用价值研究[J]. 河北医药,2022,44(8):1221-1223. [7] Rao S,Daines B,Hosseini O,et al. The utility of brain natriuretic peptide in patients undergoing an initial evaluation for pulmonary hypertension[J]. J Community Hosp Intern Med Perspect,2022,12(3):48-52. doi: 10.55729/2000-9666.1048 [8] 中华医学会妇产科学分会妊娠期高血压疾病学组. 妊娠期高血压疾病诊治指南(2015)[J]. 中华妇产科杂志,2015,50(10):721-728. doi: 10.3760/cma.j.issn.0529-567x.2015.10.001 [9] 国家基本公共卫生服务项目基层高血压管理办公室,基层高血压管理专家委员会. 国家基层高血压防治管理指南[J]. 中国循环杂志,2017,32(11):1041-1048. doi: 10.3969/j.issn.1000-3614.2017.11.001 [10] Alese M O,Moodley J,Naicker T. Preeclampsia and HELLP syndrome,the role of the liver[J]. J Matern Fetal Neonatal Med,2021,34(1):117-123. doi: 10.1080/14767058.2019.1572737 [11] Peterson J A,Sandgren K,Levine LD. Severe preterm preeclampsia: An examination of outcomes by race[J]. Am J Obstet Gynecol MFM,2020,2(4):100181. doi: 10.1016/j.ajogmf.2020.100181 [12] 刘蓉,林晓峰,魏璞,等. 子痫前期产妇产后高血压的发病情况及其危险因素分析[J]. 实用预防医学,2018,25(8):954-957. [13] Abdelazim I A,Amer O O,Shikanova S,et al. Protein/creatinine ratio versus 24-hours urine protein in preeclampsia[J]. Ginekol Pol,2022,93(12):975-979. [14] Aynaoğlu Yıldız G,Topdağı Yılmaz EP. The association between protein levels in 24-hour urine samples and maternal and neonatal outcomes of pregnant women with preeclampsia[J]. J Turk Ger Gynecol Assoc,2022,23(3):190-198. doi: 10.4274/jtgga.galenos.2022.2022-4-3 [15] 王之信,周萍. 24 h尿蛋白、胱抑素C、D-二聚体及超敏C-反应蛋白与妊娠期高血压疾病严重程度的相关性及对不良妊娠结局的预测价值[J]. 安徽医药,2022,26(8):1584-1589. doi: 10.3969/j.issn.1009-6469.2022.08.023 [16] Sukmanee J,Rothmanee P,Sriwimol W,et al. Levels of blood pressure,cardiovascular biomarkers and their correlations in women with previous pre-eclamptic pregnancy within 7 years postpartum: a cross-sectional study in Thailand[J]. BMJ Open,2022,12(6):e055534. doi: 10.1136/bmjopen-2021-055534 [17] Trejo-Soto C,Hernández-Machado A. Normalization of blood viscosity according to the hematocrit and the shear rate[J]. Micromachines (Basel),2022,13(3):357-366. doi: 10.3390/mi13030357 [18] Zhao R M,Dai H,Arias R J,et al. Direct activation of the proton channel by albumin leads to human sperm capacitation and sustained release of inflammatory mediators by neutrophils[J]. Nat Commun,2021,12(1):3855-3864. doi: 10.1038/s41467-021-24145-1 [19] 程敏,李青,李志芳. 红细胞压积及白蛋白指标评估子痫前期治疗效果的应用价值研究[J]. 河北医药,2022,44(8):1221-1223. [20] von Petersdorff-Campen K,Fischer P,Bogdanova A,et al. Potential factors for poor reproducibility of in vitro hemolysis testing[J]. ASAIO J,2022,68(3):384-393. doi: 10.1097/MAT.0000000000001577 [21] Van de Wouw J,Joles JA. Albumin is an interface between blood plasma and cell membrane,and not just a sponge[J]. Clin Kidney J,2021,15(4):624-634. [22] Nishikimi T,Nakagawa Y. B-Type Natriuretic Peptide (BNP) revisited-is BNP still a biomarker for heart failure in the angiotensin receptor/neprilysin inhibitor era?[J].Biology (Basel),2022,11(7): 1034-1042. [23] 黄钧,胡玲,佘广彤. 联合监测无创血流动力学和脑钠肽评估妊娠高血压病患者心功能的价值[J]. 江苏医药,2020,46(10):984-988. [24] Boucly A,Tu L,Guignabert C,et al. Cytokines as prognostic biomarkers in pulmonary arterial hypertension[J]. Eur Respir J,2023,61(3):2201232. doi: 10.1183/13993003.01232-2022 -






 下载:
下载:

 下载:
下载:


