Effect of Gossypol Acetate Acid on Invasion of Human Tongue Squamous Cell Carcinoma Cal-27 Cells in vitro
-
摘要:
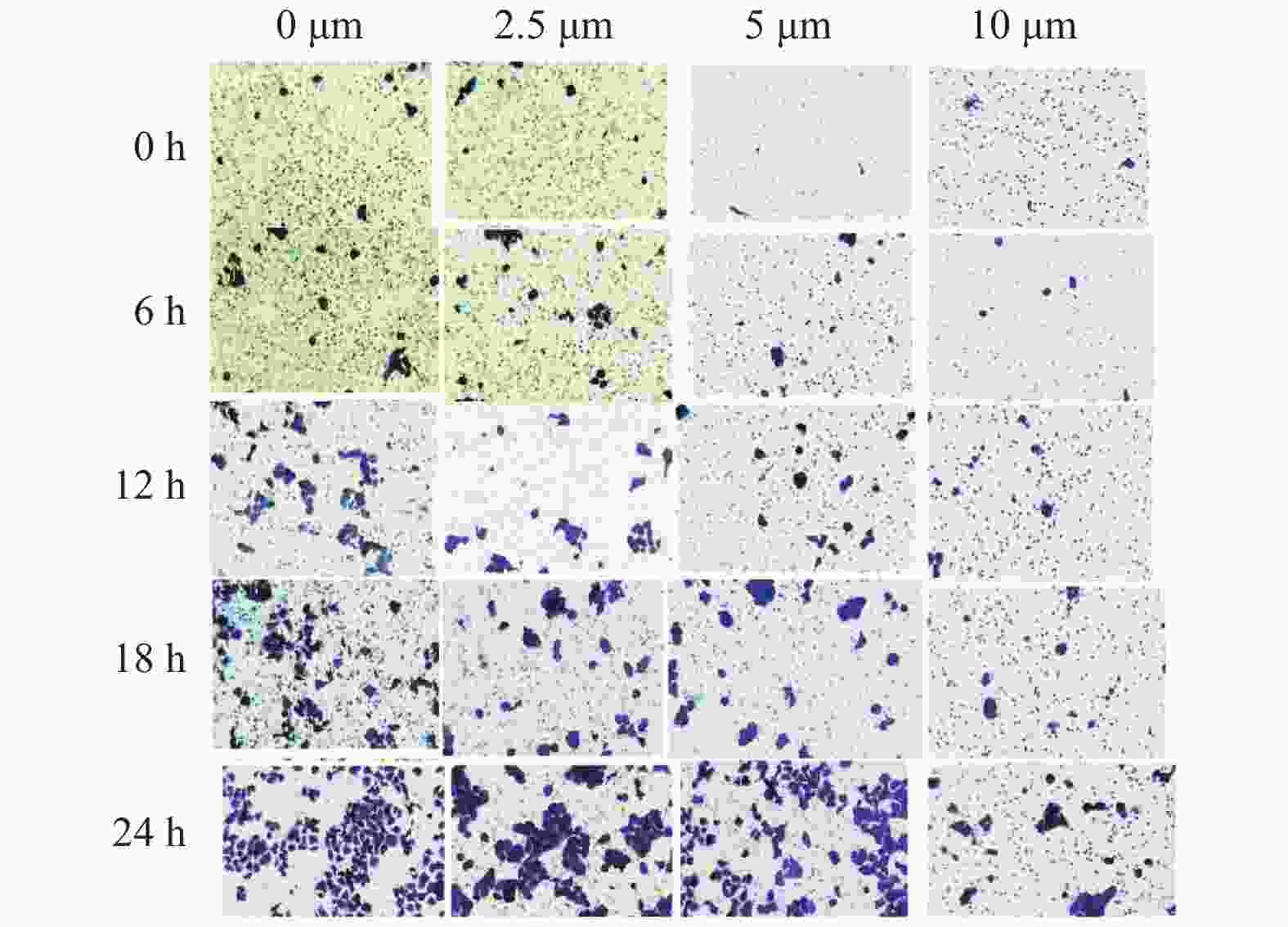
目的 研究醋酸棉酚(gossypol acetic acid,GAA)对体外培养的人舌鳞癌Cal-27细胞侵袭影响及其作用机制。 方法 制备0 μmol/L、2.5 μmol/L、5.0 μmol/L、10.0 μmol/L的GAA作用于人舌鳞癌Cal-27 细胞,通过Transwell实验检测GAA对人舌鳞癌Cal-27 细胞侵袭能力的影响;通过qRT-PCR及Western blot实验检测GAA对人舌鳞癌Cal-27 细胞基质金属蛋白酶-2(Matrix Metalloproteinase-2,MMP-2)、基质金属蛋白酶-9(Matrix Metalloproteinase-9,MMP-9)mRNA及蛋白的表达变化。 结果 Transwell实验显示:GAA对人舌鳞癌Cal-27细胞细胞侵袭产生抑制作用,实验组和对照组比较差异有统计学意义(P < 0.05);qRT-PCR实验及Western blot显示,实验组人舌鳞癌Cal-27细胞中MMP-2、MMP-9 mRNA及蛋白的表达降低,与对照组相比差异有统计学意义(P < 0.05)。 结论 GAA能抑制人舌鳞癌Cal-27细胞的侵袭性,并降低人舌鳞癌Cal-27细胞MMP-2、MMP-9的mRNA及蛋白表达水平,这可能是GAA抑制肿瘤侵袭作用的机制之一。 Abstract:Objective To observe the effect of gossypol acetate acid(GAA)on the invasion of human tongue squamous cell carcinoma Cal-27 cells in vitro. Methods GAA of different concentrations(0 μmol/L, 2.5 μmol/L, 5.0 μmol/L, 10.0 μmol/L)was acted on Cal-27 cells, Transwell experiment was employed to detect the inhibitory effect of GAA on the invasion of Cal-27 cells. The effect of gossypol acetate on MMP-2 and MMP-9 mRNA expression was quantified by qRT-PCR. The effect of gossypol acetate acid on MMP-2 and MMP-9 protein expression was analyzed by Western-Blot experiment. Results Cell Transwell chamber experiment showed that GAA could inhibit the migration of human tongue squamous cell carcinoma Cal-27 cells, and the amount of invasion decreased with the increase in concentration. The difference between the control group and the experimental group was statistically significant(P < 0.05). qRT-PCR experiment and Western-blot results showed that the expression of MMP-2 and MMP-9 mRNA and proteins was significantly reduced after Cal-27 cells of human tongue squamous cell carcinoma was treated with GAA, and the difference between the control group and the experimental group was statistically significant(P < 0.05). Conclusions GAA can reduce the invasiveness of tongue squamous cell carcinoma Cal-27 cells. And GAA can reduce the mRNA and protein expression levels of MMP-2 and MMP-9 of human tongue squamous cell carcinoma Cal-27 cells. -
表 1 MMP-2、MMP-9、GAPDH的引物序列
Table 1. Primer sequences of MMP-2,MMP-9 and GAPDH
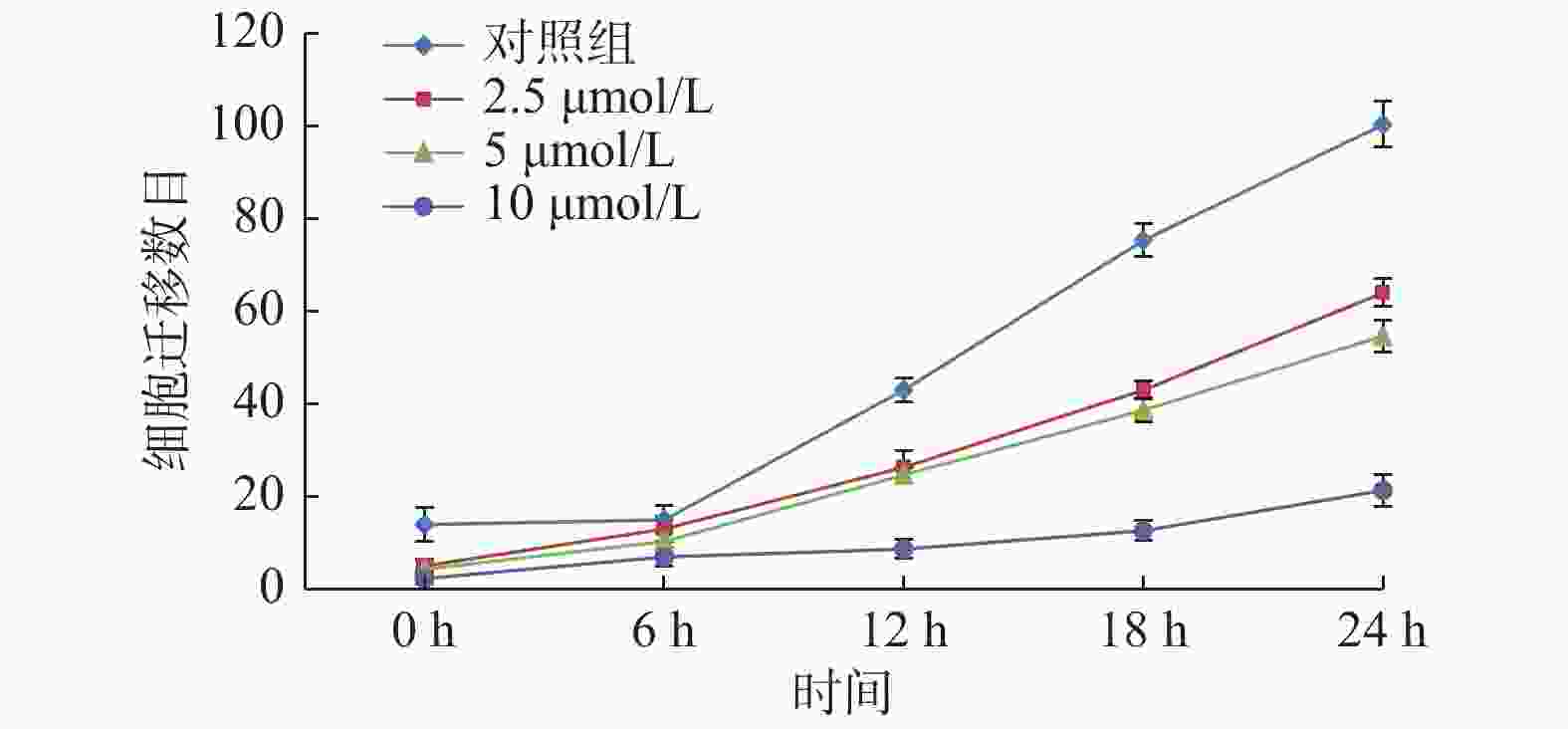
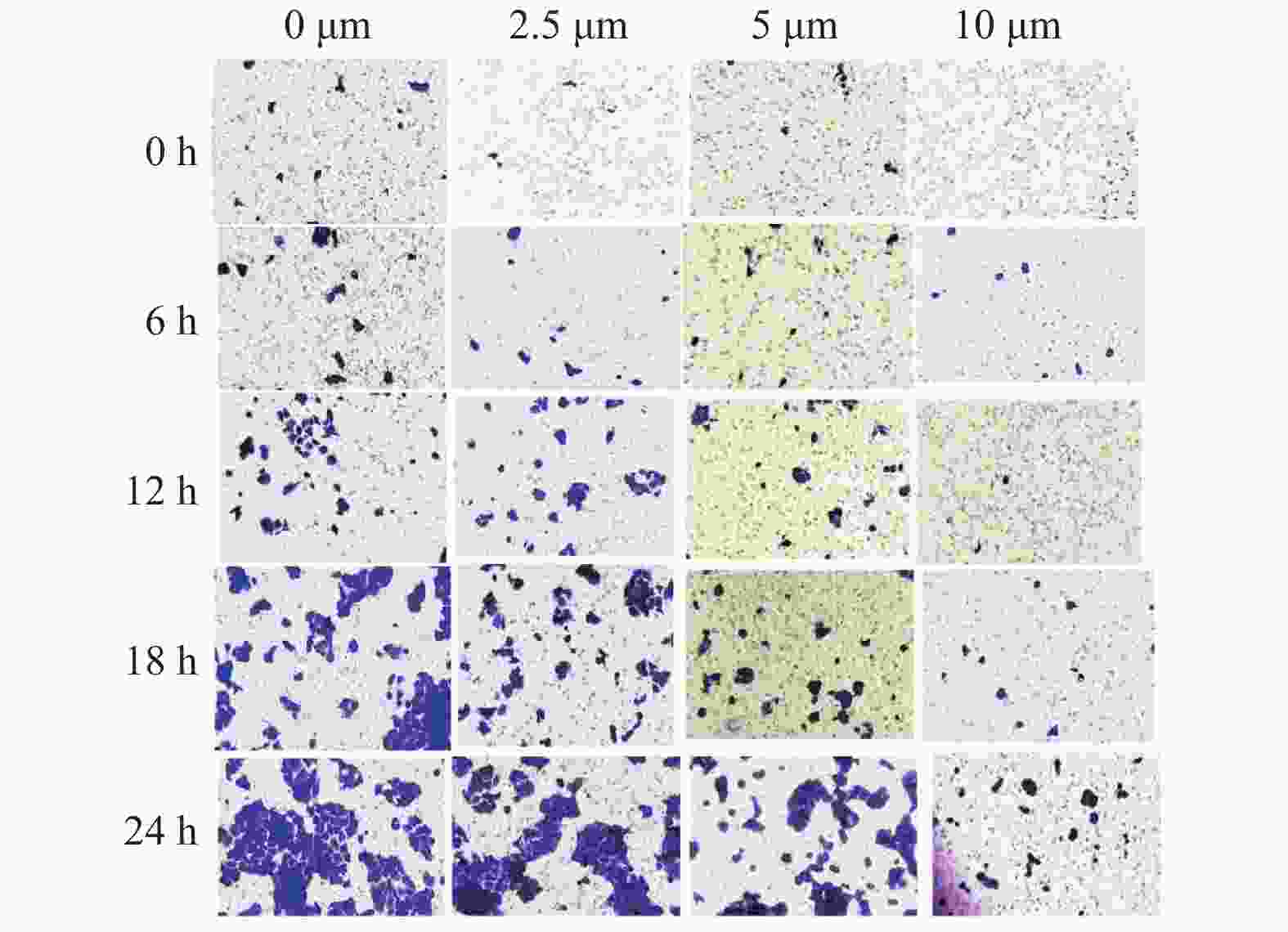
基因 引物序列 大小(bp) MMP-2(F) 5′-GAACCAGATCACATACAGGATC-3′ MMP-2(R) 5′-ATCCACTGTCTCTGGGTCCA-3′ 66 bp MMP-9(F) 5′-ACCTCAAGTGGCACCACCAC-3′ MMP-9(R) 5′-GCGGCAAGTCTTCCGAGTAG-3′ 63 bp GAPDH(F) 5′-GCCATCAATGACCCCTTCAT-3′ GAPDH(R) 5′-TTGGAACATGTAAACCATGT -3′ 51 bp 表 2 GAA对Cal-27细胞Transwell迁移个数对比(个)[(
$\bar{{{x}}}\pm {{s}}$ ,n = 3)]Table 2. Comparison of the migration of Cal-27 cell in different concentration of GAA(each)[(
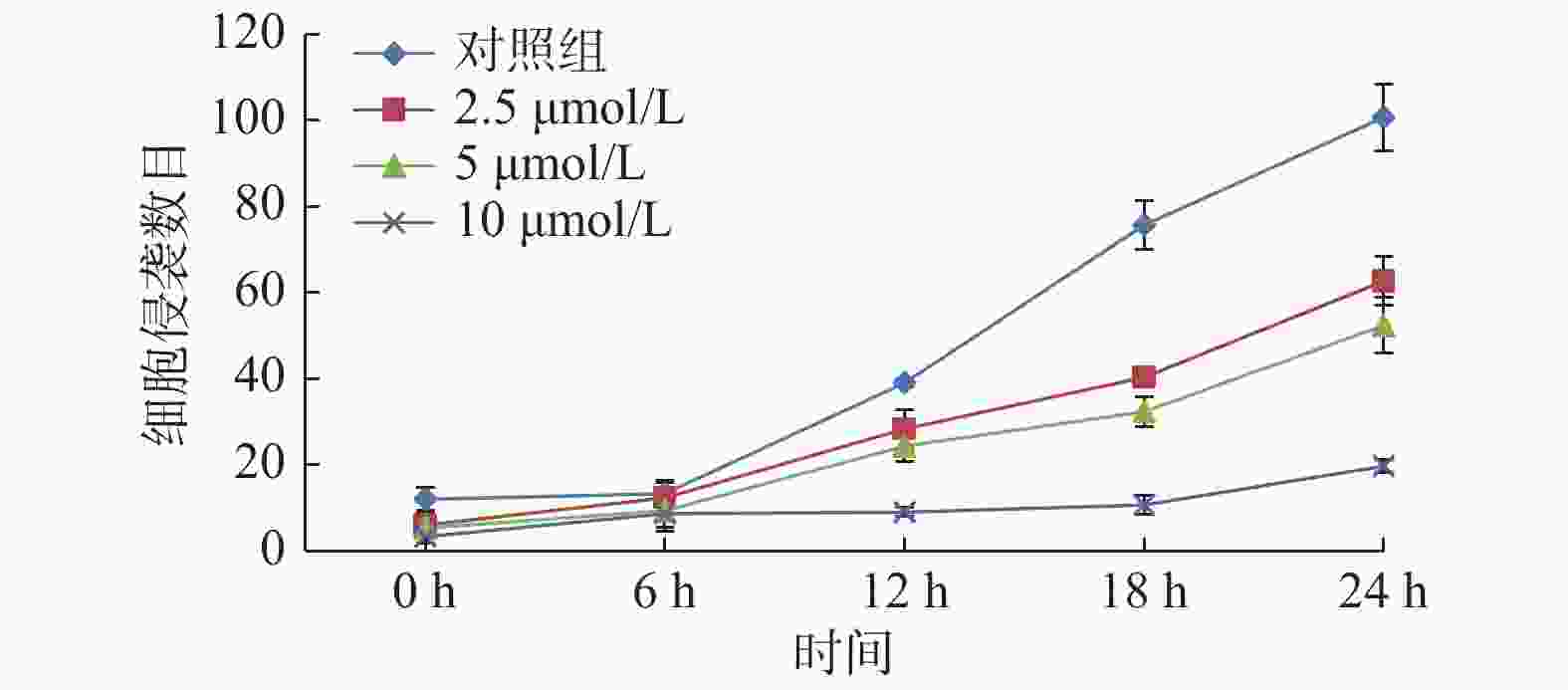
$\bar{{{x}}}\pm {{s}}$ ,n = 3)]组别 0 h 6 h 12 h 18 h 24 h 对照组 14.00 ± 3.61 15.00 ± 3.00▽ 43.00 ± 2.65▽△ 75.33 ± 3.51▽△☆ 100.33 ± 4.93▽△☆◇ 2.5 μmol/L组 5.00 ± 1.00▲ 13.00 ± 2.65▲▽ 26.33 ± 3.51▲▽△ 43.00 ± 2.00▲▽△☆ 64.00 ± 3.00▲▽△☆◇ 5 μmol/L组 4.33 ± 2.08▲★ 10.33 ± 2.52▲★▽ 24.67 ± 1.53▲★▽△ 38.67 ± 2.52▲★▽△☆ 54.67 ± 3.51▲★▽△☆◇ 10 μmol/L组 2.33 ± 1.15▲★■ 7.00 ± 2.00▲★■▽ 8.67 ± 2.08▲★■▽△ 12.67 ± 2.08▲★■▽△☆ 21.33 ± 3.51▲★■▽△☆◇ 相同作用时间与对照组比较,▲P < 0.05;相同作用时间与2.5 μmol/L比较,★P < 0.05;相同作用时间与5 μmol/L比较,■P < 0.05;相同GAA浓度作用与0 h比较,▽P < 0.05;相同GAA浓度作用与6 h比较,△P < 0.05;相同GAA浓度作用与12 h比较,☆P < 0.05;相同GAA浓度作用与18 h比较,◇P < 0.05。 表 3 GAA对Cal-27细胞Transwell侵袭个数对比(个)[(
$\bar{{{x}}}\pm {{s}}$ ),n = 3]Table 3. Comparison of the invasion of Cal-27 cell in different concentration of GAA(each) [(
$\bar{{{x}}}\pm {{s}}$ ),n = 3]组别 0 h 6 h 12 h 18 h 24 h 对照组 12.00 ± 2.65 13.33 ± 3.06▽ 39.00 ± 1.00▽△ 75.67 ± 5.69▽△☆ 100.67 ± 7.77▽△☆◇ 2.5 μmol/L组 6.00 ± 2.00▲ 12.33 ± 3.51▲▽ 28.33 ± 4.51▲▽△ 40.33 ± 1.53▲▽△☆ 62.67 ± 5.69▲▽△☆◇ 5 μmol/L组 5.33 ± 3.51▲★ 9.33 ± 4.73▲★▽ 24.33 ± 3.51▲★▽△ 32.33 ± 3.51▲★▽△☆ 52.33 ± 6.51▲★▽△☆◇ 10 μmol/L组 3.33 ± 1.53▲★■ 8.67 ± 3.06▲★■▽ 9.00 ± 1.00▲★■▽△ 10.67 ± 2.08▲★■▽△☆ 19.67 ± 1.53▲★■▽△☆◇ 相同作用时间与对照组比较,▲P < 0.05;相同作用时间与2.5 μmol/L比较,★P < 0.05;相同作用时间与5 μmol/L比较,■P < 0.05;相同GAA浓度作用与0 h比较,▽P < 0.05;相同GAA浓度作用与6 h比较,△P < 0.05;相同GAA浓度作用与12 h比较,☆p < 0.05;相同GAA浓度作用与18 h比较,◇P < 0.05。 表 4 GAA对Cal-27细胞中MMP-2、MMP-9 mRNA表达的影响(
$\bar{{{x}}}\pm {{s}}$ ,n = 9)Table 4. The mRNA expression level of MMP-2,MMP-9 in Cal- 27 cells by GAA(
$\bar{{{x}}}\pm {{s}}$ ,n = 9)组别 MMP-2 MMP-9 对照组 1.02 ± 0.24 1.01 ± 0.15 2.5 μmol/L组 0.25 ± 0.04▲ 0.83 ± 0.10▲ 5 μmol/L组 0.16 ± 0.06▲ 0.69 ± 0.03▲★ 10 μmol/L组 0.10 ± 0.03▲★ 0.46 ± 0.18▲★■ 与对照组比较,▲P < 0.05;与2.5 μmol/L比较,★P < 0.05;与5 μmol/L比较,■P < 0.05。 表 5 不同浓度GAA作用于人舌鳞癌Cal-27细胞MMP-2、MMP-9蛋白表达相对值(MMP-2、MMP-9/GAPDH)(
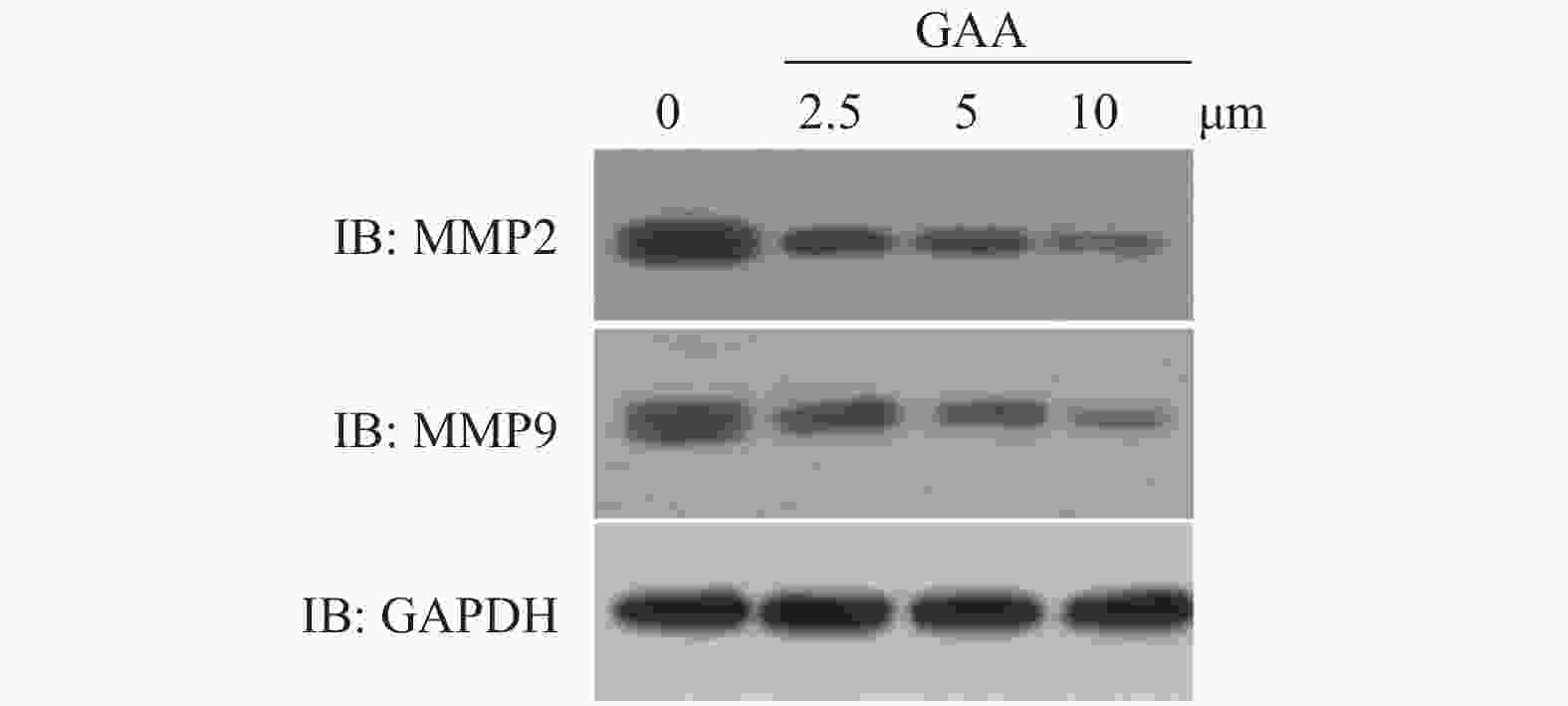
$\bar{{{x}}}\pm {{s}}$ ,n = 3)Table 5. The protein expression level of MMP-2、MMP-9 in cal- 27 cells by GAA(MMP-2、MMP-9/GAPDH) (
$\bar{{{x}}}\pm {{s}}$ ,n = 3)组别 MMP2/GAPDH MMP9/GAPDH 对照组 1.20 ± 0.02 1.20 ± 0.01 2.5 μmol/L组 0.98 ± 0.00▲ 0.91 ± 0.01▲ 5 μmol/L组 0.76 ± 0.00▲★ 0.75 ± 0.00▲★ 10 μmol/L组 0.61 ± 0.01▲★■ 0.53 ± 0.00▲★■ 与对照组比较,▲P < 0.05;与2.5 μmol/L比较,★P < 0.05;与5 μmol/L比较,■P < 0.05。 -
[1] Majchrzak E,Szybiak B,Wegner A,et al. Oral cavity and oropharyngeal squamous cell carcinoma in young adults:A review of the literature[J]. Radiol Oncol,2014,48(1):1-10. doi: 10.2478/raon-2013-0057 [2] Siegel R L,Miller K D,Jemal A. Cancer statistics,2019[J]. CA Cancer J Clin,2019,69(1):7-34. doi: 10.3322/caac.21551 [3] Siegel R L,Miller K D,Jemal A. Cancer statistics,2017[J]. CA Cancer J Clin,2017,67(1):7-30. doi: 10.3322/caac.21387 [4] Zeng Y,Ma J,Xu L,et al. Natural product gossypol and its derivatives in precision cancer medicine[J]. Curr Med Chem,2019,26(10):1849-1873. doi: 10.2174/0929867324666170523123655 [5] Gadelha I C,de Macedo M F,Oloris S C,et al. Gossypol promotes degeneration of ovarian follicles in rats[J]. ScientificWorldJournal,2014,2014:986184. [6] Kim H Y,Lee B I,Jeon J H,et al. Gossypol suppresses growth of temozolomide-resistant glioblastoma tumor spheres[J]. Biomolecules,2019,9(10):1-12. [7] Ulus G,Koparal A T,Baysal K,et al. The anti-angiogenic potential of(+/-)gossypol in comparison to suramin[J]. Cytotechnology,2018,70(6):1537-1550. doi: 10.1007/s10616-018-0247-z [8] Blanchard P,Belkhir F,Temam S,et al. Outcomes and prognostic factors for squamous cell carcinoma of the oral tongue in young adults:a single-institution case-matched analysis[J]. Eur Arch Otorhinolaryngol,2017,274(3):1683-1690. doi: 10.1007/s00405-016-4419-1 [9] Stein M N,Hussain M,Stadler W M,et al. A phase Ⅱ study of AT-101 to overcome Bcl-2--mediated resistance to androgen deprivation therapy in patients with newly diagnosed castration-sensitive metastatic prostate cancer[J]. Clin Genitourin Cancer,2016,14(1):22-27. doi: 10.1016/j.clgc.2015.09.010 [10] Cao S,Wang G,Ge F,et al. Gossypol inhibits 5alpha-reductase 1 and 3alpha-hydroxysteroid dehydrogenase:Its possible use for the treatment of prostate cancer[J]. Fitoterapia,2018,133:102-108. [11] Zhang G,Wang Z,Chen W,et al. Dual effects of gossypol on human hepatocellular carcinoma via endoplasmic reticulum stress and autophagy[J]. Int J Biochem Cell Biol,2019,113:48-57. doi: 10.1016/j.biocel.2019.05.012 [12] Guo Z,Zhao J,Song L,et al. Induction of H2AX phosphorylation in tumor cells by gossypol acetic acid is mediated by phosphatidylinositol 3-kinase(PI3K)family[J]. Cancer Cell Int,2014,14(1):141. doi: 10.1186/s12935-014-0141-5 [13] Zhao L,Niu H,Liu Y,et al. LOX inhibition downregulates MMP-2 and MMP-9 in gastric cancer tissues and cells[J]. J Cancer,2019,10(26):6481-6490. doi: 10.7150/jca.33223 [14] Aparna M,Rao L,Kunhikatta V,et al. The role of MMP-2 and MMP-9 as prognostic markers in the early stages of tongue squamous cell carcinoma[J]. J Oral Pathol Med,2015,44(5):345-352. doi: 10.1111/jop.12245 [15] Zhou W,Yu X,Sun S,et al. Increased expression of MMP-2 and MMP-9 indicates poor prognosis in glioma recurrence[J]. Biomed Pharmacother,2019,118:109369. doi: 10.1016/j.biopha.2019.109369 [16] Ciuca F I,Marasescu P C,Matei M,et al. The prognostic value of CXCR4,MMP-2 and MMP-9 in tongue squamous carcinoma[J]. Rom J Morphol Embryol,2019,60(1):59-68. -





 下载:
下载: