Clinical Study of Chemotherapy Combined with CIK Cell Immunotherapy for Non-small Cell Lung Cancer
-
摘要:
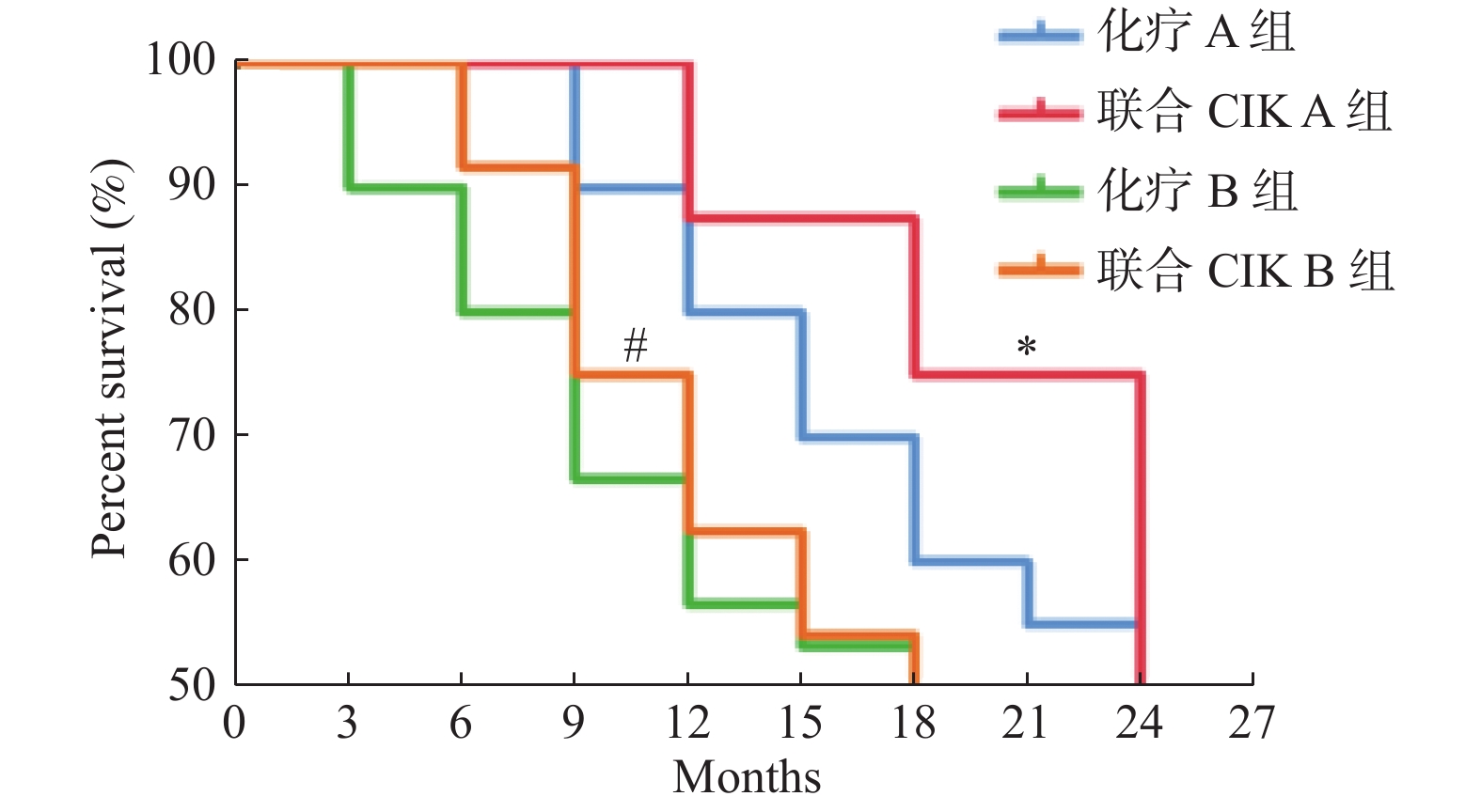
目的 评价化疗联合CIK(cytokine-induced killer)细胞免疫疗法对非小细胞肺癌NSCLC早期、晚期患者的临床疗效。 方法 收集2013年1月至2016年12月昆明医科大学第三附属医院收治NSCLC患者122例为研究对象,按患者病程分为Ⅰ-Ⅱ期和Ⅲ-Ⅳ期,将其随机分入4个受试组:化疗A组(n = 30),联合CIK A组(n = 31),化疗B组(n = 30),联合CIK B组(n = 31)。其中,受试组采用化疗方法均为吉西他滨联合顺铂治疗(GP)。评价患者临床疗效、生存质量和外周血淋巴细胞和细胞因子变化。 结果 与化疗组比较,联合CIK治疗组显著提高ORR值和DCR值,分别是(67.74%,45.16%)和(74.19%,64.52%)(P < 0.05);提高KPS评分和ECOG评分,分别是(80.65%,58.06%)和(80.65%,70.97%)( P < 0.05);提高患者外周血中CD3 +、CD8+淋巴细胞亚群比率及CIK细胞比率(P < 0.05);提高患者外周血中IFN-γ,TNF-α、IL-4、IL-6细胞因子表达水平( P < 0.05);延长患者生存期( P < 0.05)。与联合CIK A组(Ⅰ-Ⅱ期)比较,联合CIK B组(Ⅲ-Ⅳ期)患者外周血中CD3 +、CD4+、CD8+、CD4+/CD8+T淋巴细胞亚群、NK细胞比率、CIK细胞比率均显著提高(P < 0.05),维持和调控增强外周血中IFN-γ,TNF-α、IL-2、IL-4、IL-6细胞因子表达水平( P < 0.05)。 结论 化疗联合CIK细胞治疗能显著提高不同阶段NSCLC患者临床疗效、延长生存期、提高近期生存质量,提高化疗期患者体内免疫细胞持续对抗肿瘤的细胞毒性和免疫应答能力,尤其对晚期患者的疗效更佳。 Abstract:Objective To evaluate the clinical efficacy of chemotherapy combined with CIK (cytokine-induced killer)cell immunotherapy on early and advanced stages non small cell lung cancer (NSCLC)patients. Methods A total of 122 NSCLC patients admitted to the Third Affiliated Hospital of Kunming Medical University from 2013 to 2016, according to the course of disease, the patients divided into early stage (stage I-II)and late stage (stage III-IV); and were randomly divided into four groups: chemotherapy a group (n = 30), combined with CIK a group (n = 31), chemotherapy B group (n = 30), combined with CIK B group (n = 31). Among them, gemcitabine combined with cisplatin (GP)was used in all the groups. The clinical efficacy, quality of life and changes of peripheral blood lymphocytes and cytokines were evaluated. Results Compared with the chemotherapy group, the ORR and DCR values were significantly increased (67.74%, 45.16%)and (74.19%, 64.52%)respectively (P < 0.05), KPS and ECOG scores were significantly increased (80.65%, 58.06%)and (80.65%, 70.97%) respectively ( P < 0.05), in the combined CIK groups. The ratio of CD3 +, CD8+ lymphocytes and CIK cells in patients' peripheral blood was significantly increased (P < 0.05)and the expression level of IFN - γ, TNF - α, IL-4 and IL-6 cytokines in patients' peripheral blood was significantly increased ( P < 0.05)and the survival period of patients was prolonged ( P < 0.05)in the combined CIK groups. Compared with the combined CIK A group (early stage), the ratio of CD3 +, CD4+, CD8+, CD4+/CD8+ T lymphocytes, NK cell ratio and CIK cell ratio in the peripheral blood of the patients were significantly increased, and the expression levels of IFN - γ, TNF - α, IL-2, IL-4 and IL-6 cytokines in the peripheral blood were maintained and enhanced in the combined CIK B group (advanced stage)(P < 0.05). Conclusion Chemotherapy combined with CIK cell therapy can significantly improve the clinical efficacy, prolong the survival period, and improve the short-term quality of life of NSCLC patients in different stages, and significantly improve the ability of immune cells in vivo to continuously resist tumor cytotoxicity and immune response, especially for patients in advanced stage. -
Key words:
- CIK cell immunotherapy /
- Non-small cell lung cancer /
- Chemotherapy /
- Combined therapy
-
表 1 各受试组患者统计情况(
$ \bar x \pm s$ )Table 1. Statistics of patients in each group(
$ \bar x \pm s$ )组别 n 性别比率 平均年龄(岁) TNM分期人数(n) 男∶女 I II III IV 化疗A组 30 15∶15 63.67 ± 9.54 16 14 联合CIK A组 31 20∶11 65.23 ± 10.61 18 13 化疗B组 30 18∶12 63.40 ± 13.29 10 20 联合CIK B组 31 18∶13 66.87 ± 11.21 7 24 表 2 各受试组临床近期疗效情况
Table 2. The short-term clinical efficacy in all groups
组别 n CR(n) PR(n) SD(n) PD(n) ORR(%) DCR(%) 化疗A组 30 16 0 5 9 53.33 70.00 联合CIK A组 31 21 0 2 8 67.74* 74.19 t 17.471 8.568 P 0.036 0.074 化疗B组 30 0 9 6 15 30.00 50.00 联合CIK B组 31 5 9 6 11 45.16# 64.52# t 14.725 16.054 P 0.043 0.040 与化疗A组比较,*P < 0.05;与化疗B组比较, #P < 0.05。 表 3 治疗后各受试组KPS评分变化
Table 3. Changes of KPS score in all groups after treatments
组别 n 提高(n) 稳定(n) 下降(n) 提高率(%) 化疗A组 30 0 22 8 73.33 联合CIK A组 31 0 25 6 80.65* t 13.727 P 0.046 化疗B组 30 0 14 16 46.67 联合CIK B组 31 0 18 13 58.06# t 18.50 P 0.034 与化疗A组比较,*P < 0.05;与化疗B组比较, #P < 0.05。 表 4 治疗后各受试组ECOG评分变化
Table 4. The changes of ECOG score in all groups after treatments
组别 n 提高(n) 稳定(n) 下降(n) 提高率(%) 化疗A组 30 0 20 10 66.67 联合CIK A组 31 0 25 6 80.65* t 16.217 P 0.039 化疗B组 30 0 16 14 53.33 联合CIK B组 31 0 22 9 70.97# t 22.538 P 0.028 与化疗A组比较,*P < 0.05;与化疗B组比较, #P < 0.05。 表 5 各受试组外周血T淋巴细胞亚群比率变化(
${\bar{ x}}$ ± s)Table 5. The changes of T lymphocyte subsets ratios in peripheral blood in each group(
${\bar{ x}}$ ± s)组别
T淋巴细胞亚群比率时间点 化疗A组(n = 30) 联合CIK A组(n = 31) 化疗B组(n = 30) 联合CIK B组(n = 31) CD3+(%) 治疗前 68.33 ± 11.73 66.69 ± 7.6 67.65 ± 8.99 67.68 ± 12.71 治疗后 73.25 ± 12.06∆ 69.87 ± 9.57*∆ 75.39 ± 8.23∆ 72.38 ± 5.99∆# CD4+(%) 治疗前 41.28 ± 5.94 40.9 ± 4.07 40.39 ± 9.21 41.66 ± 8.55 治疗后 37.73 ± 8.44∆ 33.48 ± 8.24*∆ 34.27 ± 7.33∆ 39.45 ± 8.62# CD8+(%) 治疗前 21.77 ± 6.87 22.31 ± 7.78 21.38 ± 9.28 21.58 ± 8.68 治疗后 20.25 ± 8.41 25.56 ± 8.03*∆ 20.43 ± 8.46 29.84 ± 9.42∆# CD4+/CD8+ 治疗前 2.05 ± 0.55 1.84 ± 0.38 1.93 ± 0.86 1.96 ± 0.82 治疗后 1.75 ± 0.76∆ 1.45 ± 1.17*∆ 1.79 ± 0.75 1.62 ± 1.20 NK(%) 治疗前 17.28 ± 11.28 16.39 ± 6.22 15.35 ± 6.63 16.88 ± 7.40 治疗后 15.15 ± 11.09 15.99 ± 9.42 13.62 ± 6.06 16.41 ± 7.36# CIK(%) 治疗前 1.22 ± 0.85 1.11 ± 0.94 1.35 ± 1.33 1.20 ± 1.14 治疗后 1.32 ± 0.82 2.32 ± 2.09*∆ 1.41 ± 1.33 1.92 ± 1.31∆# 与治疗前比较,∆P < 0.05;与化疗A组比较, *P < 0.05;与化疗B组比较, #P < 0.05。 表 6 各受试组外周血细胞因子表达水平[(
${\bar{ x}}$ ± s),Pg/mL]Table 6. The expression changes of cytokines in peripheral blood of groups[(
${\bar{ x}}$ ± s),Pg/mL]组别
细胞因子时间点 化疗A组(n = 30) 联合CIK A组(n = 31) 化疗B组(n = 30) 联合CIK B组(n = 31) IFN-γ 治疗前 65.98 ± 34.52 66.45 ± 27.12 68.98 ± 30.83 70.98 ± 43.12 治疗后 58.69 ± 14.18∆ 70.06 ± 30.25*∆ 56.32 ± 30.47∆ 74.13 ± 43.57∆# TNF-α 治疗前 31.51 ± 7.04 27.25 ± 4.25 28.02 ± 18.57 29.9 ± 13.35 治疗后 24.34 ± 10.06∆ 27.49 ± 14.36* 22.36 ± 19.03∆ 25.63 ± 18.87∆# IL-10 治疗前 26.06 ± 3.14 28.9 ± 4.32 27.95 ± 18.16 26.63 ± 12.27 治疗后 15.63 ± 8.99∆ 15.26 ± 10.15∆ 18.88 ± 15.53∆ 25.37 ± 16.97# IL-6 治疗前 18.82 ± 3.14 19.1 ± 5.69 20.96 ± 15.32 22.37 ± 10.83 治疗后 10.36 ± 5.98∆ 23.61 ± 15.39*∆ 23.25 ± 15.42∆ 26.65 ± 10.73∆# IL-4 治疗前 19.98 ± 10.21 18.23 ± 15.42 23.24 ± 14.61 25.4 ± 17.62 治疗后 20.69 ± 10.69 14.68 ± 11.49*∆ 27.36 ± 15.39∆ 30.15 ± 19.38∆# IL-2 治疗前 30.52 ± 14.91 25.65 ± 11.15 33.67 ± 16.05 27.29 ± 19.33 治疗后 26.94 ± 14.16∆ 25.98 ± 15.71 25.63 ± 11.91∆ 20.21 ± 25.37∆# 与治疗前比较,∆P < 0.05;与化疗A组比较, *P < 0.05;与化疗B组比较, #P < 0.05。 -
[1] Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA: A Cancer Journal for Clinicians, 2018, 68(6): 394-424. doi: 10.3322/caac.21492 [2] 佚名. 2018年全国最新癌症报告: 每分钟有7人被确诊为癌症[J], 新民周刊, 2018, 5(14): 53~54. [3] Jemal A, Siegel R, Ward E, et al. Cancer statistics[J]. CA Cancer J Clin. 2007, 57(1): 43-66. doi: 10.3322/canjclin.57.1.43 [4] Black R C, Khurshid H. NSCLC: an update of driver mutations, their role in pathogenesis and clinical significance[J]. Rhode Island Med J. 2015, 98(10): 25-28. [5] Williams M, Liu Z W, Hunter A, et al. An updated systematic review of lung chemo - radiotherapy using a new evidence aggregation method[J]. Lung Cancer. 2015, 87(3): 290-295. doi: 10.1016/j.lungcan.2014.12.004 [6] Verma R, Foster R E, Horgan K, et al. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer[J]. Breast Cancer Res. 2016, 18(1): 10. doi: 10.1186/s13058-015-0669-x [7] 戴秀梅, 魏卫, 袁保兰. 吉非替尼治疗前后肺腺癌患者外周血T淋巴细胞亚群检测的临床意义[J]. 中国实用医刊, 2012, 39(12): 91-92. doi: 10.3760/cma.j.issn.1674-4756.2012.08.044 [8] Chen D, Sha H H, Hu T M, et al. Cytokine-induced killer cells as a feasible adoptive immunotherapy for the treatment of lung cancer[J]. Cell Death and Disease, 2018, 9(3): 366. doi: 10.1038/s41419-018-0404-5 [9] 张志强, 马怡, 劣迹艺人, 等. 实体肿瘤治疗效果评价标准的研究进展[J]. 中国临床实用医学, 2019, 10(2): 71-73. [10] Khanna P, Blais N, Gaudreau P O, et al. Immunotherapy comes of age in lung cancer[J]. Clinical Lung Cancer, 2017, 18(1): 13-22. doi: 10.1016/j.cllc.2016.06.006 [11] Zhu J, Li R, Tiselius E, et al. Immunotherapy (Excluding checkpoint inhibitors) for stage I to III non - small cell lung cancer treated with surgery or radiotherapy with curative intent[J]. Cochrane Database of Systematic Reviews, 2017, 12(12): CD011300. [12] Zhang J, Zhu L, Du H, et al. Autologous cytokine - induced killer cell therapy in lung cancer patients: a retrospective study[J]. Biomed Pharmacother, 2015, 70: 248-252. doi: 10.1016/j.biopha.2014.12.025 [13] Shapouri M A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease[J]. Journal of Cellular Physiology, 2018, 233(9): 6425-6440. doi: 10.1002/jcp.26429 [14] Shang N, Figini M, Shangguan J, et al. Dendritic cells based immunotherapy[J]. American Journal of Cancer Research, 2017, 7(10): 2091-2102. [15] Staedtke V, Bai R Y, Kim K, et al. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome[J]. Nature. 2018, 564(7735): 273-277. [16] Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells[J]. Nat Med. 2018, 24(6): 739-748. doi: 10.1038/s41591-018-0036-4 [17] Zhang L, Wang J, Wei F, et al. Profiling the dynamic expression of checkpoint molecules on cytokine-induced killer cells from non-small-cell lung cancer patients[J]. Oncotarget. 2016, 7(28): 43604-43615. doi: 10.18632/oncotarget.9871 [18] Li D, Li C, Zhang K X, et al. CIK may enhance the lethal effect of cisplatin on the lung adenocarcinoma cell line A549[J]. Chin J Clin Lab Sci, 2013, 31(12): 915-918. [19] Zhong R, Han B, Zhong H. A prospective study of the efficacy of a combination of autologous dendritic cells, cytokine-induced killer cells, and chemotherapy in advanced non-small cell lung cancer patients[J]. Tumor Biology, 2014, 35(2): 987-994. doi: 10.1007/s13277-013-1132-1 [20] Zhang L, Xu Y, Shen J, et al. Feasibility study of DCs/CIKs combined with thoracic radiotherapy for patients with locally advanced or metastatic non-small-cell lung cancer[J]. Radiation Oncology, 2016, 11(1): 1-8. doi: 10.1186/s13014-015-0579-1 -






 下载:
下载: