Process Studies on the Surface Modification of Coral Hydroxyapatite
-
摘要:
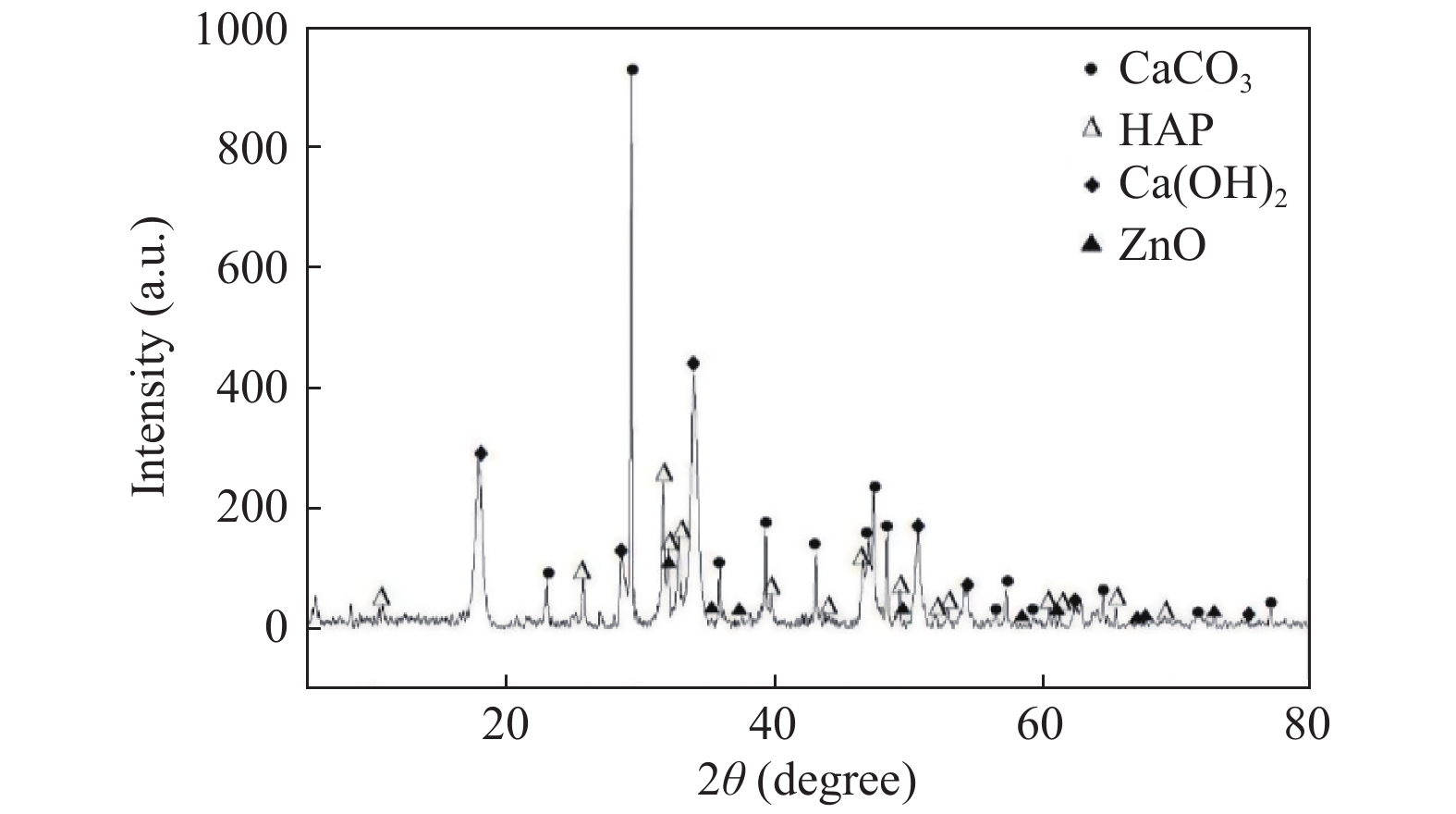
目的 探究纳米氧化锌(nmZnO)在不同条件下改性珊瑚羟基磷灰石(CHA)的工艺研究。 方法 在弱酸环境内,温度70 ℃条件下,采用硝酸锌溶胶-凝胶法改性珊瑚羟基磷灰石,通过超声、旋转搅拌、干燥、煅烧获得白色颗粒状多孔复合材料,应用扫描电子显微镜观察改性后材料表面特征。 结果 在不同原料配比条件下,nmZnO颗粒在CHA表面的分布及粒径大小存在差异。热处理过程中,保温温度及保温时间的改变会导致材料的除碳效果及结构完整性发生变化。 结论 利用硝酸锌溶胶-凝胶法可以对珊瑚羟基磷灰石进行表面改性,纳米氧化锌粒径小于100纳米,纳米粒子的团聚问题得以解决。 Abstract:Objective To explore the process of modifying coral hydroxyapatite by nmZnO under the different conditions. Methods Coral hydroxyapatite was modified by zinc nitrate sol-gel method at 70 ℃ in weak acid environment. White granular porous composite materials were obtained by ultrasonic, rotary stirring, drying and calcination. The surface characteristics of the modified materials were observed by scanning electron microscope. Results The results showed that the distribution and size of nmzno particles on the coral hydroxyapatite surface were different under the different raw material ratios. In the process of heat treatment, different holding temperature and holding time would lead to the change of carbon removal effect and structural integrity of materials. Conclusion Coral hydroxyapatite surface can be modified by zinc nitrate sol-gel method. The particle size of nano zinc oxide is less than 100 nanometers. The agglomeration problem of nano-particles is solved. -
Key words:
- Coral hydroxyapatite /
- Zinc oxide /
- Modification /
- Antibacterial property
-
表 1 复合材料热处理后情况
Table 1. Changes of composites after heat treatment
保温温度(℃) 保温1 h 保温3 h 保温5 h 560 色黑,原结构未破坏 色灰黑,原结构未破坏 色灰白,原结构未破坏 580 色灰,原结构未破坏 色灰白,原结构未破坏 色白,原结构未破坏 600 色灰白,原结构未破坏 色白黄,原结构未破坏 色白,原结构破坏 -
[1] Xu Ling,Lv Kaige,Zhang Wenjie,et al. The healing of critical-size calvarial bone defects in rat with rhPDGF-BB,BMSCs,and β-TCP scaffolds[J]. Journal of Materials Science,2012,23(4):1073-1084. [2] Guo Jun,Meng Zhaosong,Chen Gang,et al. Restoration of critical-size defects in the rabbit mandible using porous nanohydroxyapatite-polyamide scaffolds[J]. Tissue Engineering,Part A,2012,18(11-12):1239-1252. [3] Rentsch Claudia,Rentsch Barbe,Breier Annette,et al. Long-bone critical-size defects treated with tissue-engineered polycaprolactone-co-lactide scaffolds:a pilot study on rats[J]. Journal of Biomedical Materials Research,Part A,2010,95(3):964-972. [4] N. Mokbel,C. Bou Serhal,G. Matni,et al. Healing patterns of critical size bony defects in rat following bone graft[J]. Oral and Maxillofacial Surgery,2008,12(2):73-78. [5] Huo K,Zhang X,Wang H. Osteogenic activity and antibacterial effects on titanium surfaces modified with zn-incorporated nanotube arrays[J]. Biomaterials,2013,34(13):3467-3478. [6] Kasraei S,Sami L,Hendi S. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on streptococcus mutans and lactobacillus[J]. Restor Dent Endod,2014,39(2):109-114. [7] 孙士家. HAPw/nmZnO-nmCaO抗菌骨修复材料的制备及性能研究[D]. 昆明: 昆明医科大学(硕士学位论文), 2017. [8] Malat T A,Glombitza M,Dahmen J,et al. The use of bioactive glass S53P4 as bone graft substitute in the treatment of chronic osteomyelitis and infected non-unions-a retrospective study of 50 patients[J]. Z Orthop Unfall,2018,156(2):152-159. [9] Tao Cheng,Haiyun Qu,Guoyou Zhang,et al. Osteogenic and antibacterial properties of vancomycin-laden mesoporous bioglass/PLGA composite scaffolds for bone regeneration in infected bone defects[J]. Artificial Cells,Nanomedicine,and Biotechnology,2018,46(8):1935-1947. [10] Wei S,Jian C,Xu F,et al. Vancomycin-impregnated electrospun polycaprolactone(PCL)membrane for the treatment of infected bone defects:An animal study[J]. J Biomater Appl,2018,32(9):1187-1196. [11] 刘丹华,张晓伟,张翀. 抗生素滥用与超级细菌[J].国外医药(抗生素分册),2019,40(1):1-4. doi: 10.3969/j.issn.1001-8751.2019.01.002 [12] 谢晓亮,白凡. 科学家揭示细菌耐药性产生分子机制[J].微生物学通报,2016,43(5):1164. [13] Lindner T,Kanakaris N K,Marx B,et al. Fractures of the hip and osteoporosis:the role of bone substitutes[J]. Bone Joint Surg Br,2009,91(3):294-303. [14] 季海波. 细菌对抗菌药物的耐药性分析[J].中外医疗,2018,37(2):92-195. [15] 项荣,丁栋博,范亮亮,等. 氧化锌的抗菌机制及其安全性研究进展[J].中国组织工程研究,2014,18(3):470-475. doi: 10.3969/j.issn.2095-4344.2014.03.023 [16] Roy D M,Linnehan S K. Hydroxyapatite formed from coral skeletal carbonate by hydrothermal exchange[J]. Nature,1974,247(5438):220-222. [17] Fu Kun,Xu Qingguo,Czernuszka Jan,et al. Characterization of a biodegradable coralline hydroxyapatite/calcium carbonate composite and its clinical implementation[J]. Biomedical Materials,2013,8(6):1327-1329. [18] 陈泽鹏,章莹,林泽枫,等. 硫酸葡聚糖/重组人BMP-2/壳聚糖复合微球联合珊瑚羟基磷灰石人造骨修复大段骨缺损影像学评价[J].中国修复重建外科杂志,2017,31(11):1384-1389. [19] 刘畅. 不同置换率珊瑚羟基磷灰石体外降解研究[D]. 郑州: 郑州大学(硕士学位论文), 2016. [20] Hak D J. The use of osteoconductive bone graft substitutes in orthopaedic trauma[J]. The Journal of the American Academy of Orthopaedic Surgeons,2007,15(9):525-536. [21] 孙士家,赵强,袁艳波,等. pH值对HAPw-nmZnO-nmCaO复合生物材料形貌影响的研究[J].口腔医学研究杂志,2017,33(6):585-588. [22] 曾鲜丽. 纳米氧化锌抗真菌机制的研究[D]. 株洲: 湖南工业大学(硕士学位论文), 2017. [23] Mladenovic Z,Sahlin-Platt A,Andersson B,et al. In vitro study of the biological interface of bio-oss:implications of the experimental setup[J]. Clinical Oral Implants Research,2013,24(3):329-335. [24] El-Ghannam A,Ning C Q. Effect of bioactive ceramic dissolution on the mechanism of bone mineralization and guided tissue growth in vitro[J]. Journal of Biomedical Materials Research. Part A,2006,76(2):386-397. [25] Beck George R. Inorganic phosphate as a signaling molecule in osteoblast differentiation[J]. Journal of Cellular Biochemistry,2003,90(2):234-243. -






 下载:
下载: