Effect of Tumor Antigen Loaded DC-CIK on Microenvironment of Hepatocellular Carcinoma
-
摘要:
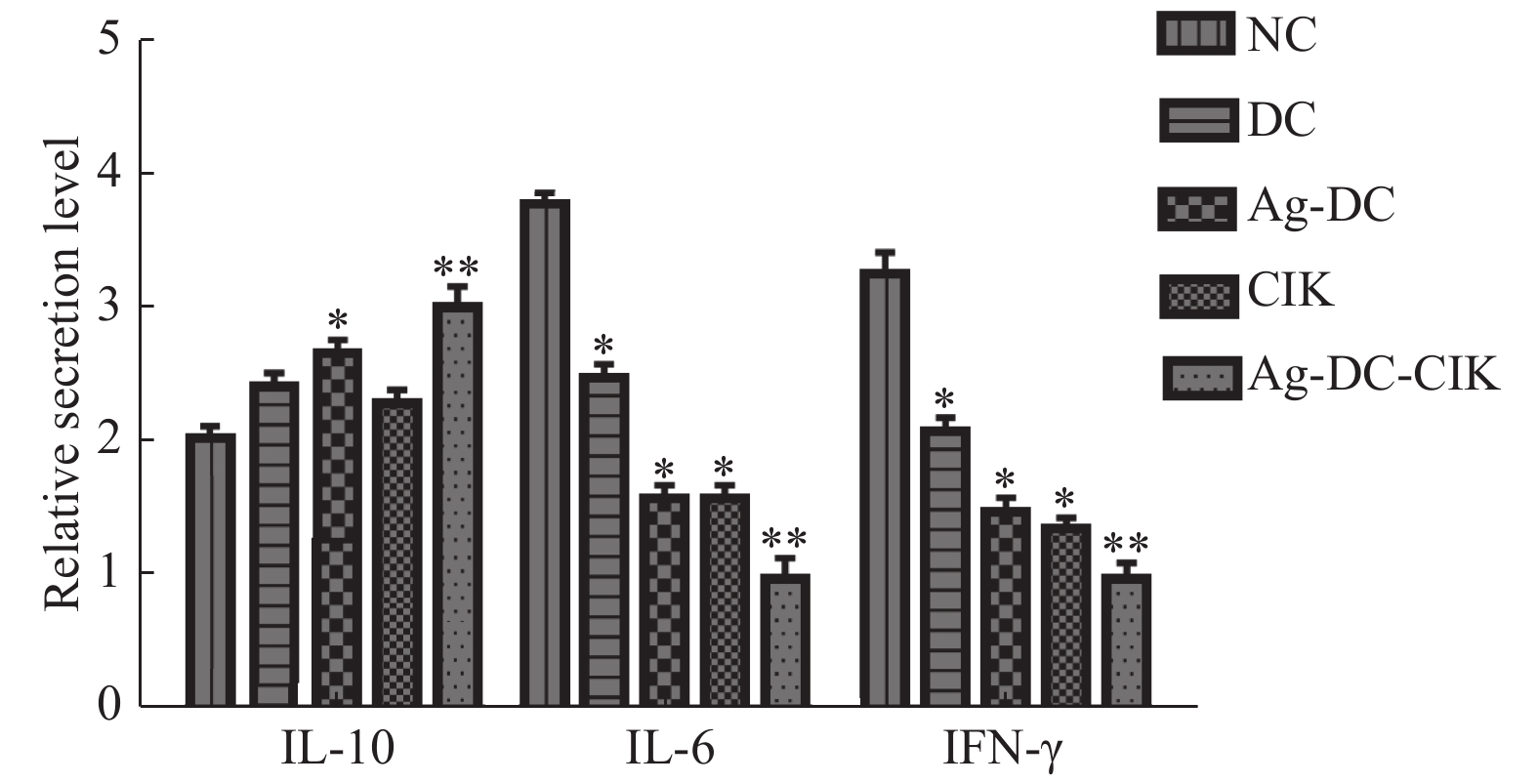
目的 探讨负载肝癌抗原树突状细胞(dendritic cell,DC)联合细胞因子诱导杀伤细胞(cytokine-induced killer,CIK)对肝细胞性肝癌(hepatocellular carcinoma,HCC)微环境的影响。 方法 采用手术切除HCC组织制备HCC抗原,采集健康志愿者单个核细胞,诱导并分离出DC,CIK,按是否加入HCC抗原及共培养将细胞分为DC、Ag-Dc、CIK、Ag-DC-CIK四组,流式细胞仪测定各组细胞表型后,ELISA检测IL-6、IL-10及IFN-γ水平;免疫细胞化学SP法检测血管内皮生长因子(vascular endothelial growth factor,VEGF)的表达;RT-PCR检测基质金属蛋白酶(matrix metalloproteinase,MMP)-2,MMP-3,MMP-9mRNA表达水平。 结果 负载抗原后DC组CD80及CD86表达阳性率,Ag-DC-CIK组CD3+CD56+、CD8+CD56+双阳性细胞均显著升高(P < 0.05); Ag-DC-CIK组IL-6及IFN-γ浓度显著低于其余各组;而IL-10浓度最高(P < 0.05);Ag-DC-CIK组VEGF及MMP-2、MMP-3、MMP-9mRNA表达水平均显著下降(P < 0.05)。 结论 在DC负载自身抗原的基础上,与CIK共培养,可显著增强DC及CIK活性,有效降低HePG2微环境中血管形成,炎症反应及纤维化相关因子表达。 -
关键词:
- 肝细胞肝癌 /
- 微环境 /
- 树突状细胞 /
- 细胞因子诱导杀伤细胞
Abstract:Objective To study the effect of tumor antigen loaded DC-CIK on the microenvironment of hepatocellular carcinoma. Methods The antigen of HCC coming from resected HCC and peripheral blood mononuclear cells were isolated from healthy donors. The cells were divided into 4 groups as DC, Ag-DC, CIK and Ag-DC-CIK according to the loading of HCC antigen or coculture of DC-CIK or not. Cell phenotype of 4 groups were evaluated by FCM. Furthermore, levels of IL-6, IL-10 and IFN-γ; expression of VEGF and MMP-2、MMP-3、MMP-9mRNA were observed. Results The contents of CD80, CD86 in group Ag-DC and CD3+CD56+, CD8+CD56+ double positive cells in group Ag-DC-CIK elevated significantly(P < 0.05). The level of IL-6 and IFN-γdecreased significantly in group Ag-DC-CIK(P < 0.05). Expression of VEGF and MMP-2, MMP-3, MMP-9mRNA also reduced significantly in group Ag-DC-CIK(P < 0.05). Conclusion Coculture of DC and CIK on the basis of loading HCC antigen shows the stronger activity and reduces the expression of relating factors about inflammation, angiopoiesis and fibrosis in HePG2 microenvironment. -
Key words:
- Hepatocellular carcinoma /
- Microenvironment /
- Dendritic cell /
- Cytokine-induced killer
-
表 1 两组DC表面标志分子表达比较(
$\bar x \pm s $ )Table 1. Comparison of surface marker expression between DC and Ag-DC(
$\bar x \pm s $ )表面标记物 分组 t P DC Ag-DC CD80 52.07 ± 0.78 63.47 ± 0.93 −134.5 0.020 CD86 43.20 ± 0.95 61.33 ± 0.78 −150.7 0.016 表 2 各组CIK细胞表型比较(
$\bar x \pm s $ )Table 2. Comparison of immune phenotypes in different CIK groups(
$\bar x \pm s $ )细胞表型 分组 F P CIK DC-CIK Ag-DC-CIK CD3+CD8+ 48.37 ± 2.09 59.35 ± 1.28 76.27 ± 3.32 407.29 0.013 CD3+CD56+ 16.43 ± 1.21 35.51 ± 2.03 57.73 ± 2.67 356.19 0.021 -
[1] Mak L Y. Editorial:Can hepatitis B core-related antigen be the new biomarker for hepatocellular carcinoma in nucleoside analogue-treated chronic hepatitis B?[J]. Aliment PharmacolTher,2019,49(7):954-955. doi: 10.1111/apt.15143 [2] Zhang J,Li H,Gao D,et al. A prognosis and impact factor analysis ofDC-CIK cell therapy for patients with hepatocellular carcinoma undergoing postoperative TACE[J]. Cancer Biol Ther,2018,19(6):475-483. doi: 10.1080/15384047.2018.1433501 [3] Zhang B,Zhang B,Zhang Z,et al. 42,573 cases of hepatectomy in China:a multicenter retrospective investigation[J]. Sci China Life Sci,2018,61(6):660-670. doi: 10.1007/s11427-017-9259-9 [4] Schmidt N,Thimme R. Role of immunity in pathogenesis and treatment of hepatocellular carcinoma[J]. Dig Dis,2016,34(4):429-437. doi: 10.1159/000444558 [5] Yoon J S,Song B G,Lee J H,et al. Adjuvant cytokine-induced killer cell immunotherapy for hepatocellular carcinoma:A propensity score-matched analysis of real-world data[J]. BMC Cancer,2019,19(1):523. doi: 10.1186/s12885-019-5740-z [6] Park R,Eshrat F,Al-Jumayli M,et al. Immuno-Oncotherapeutic approaches in advanced hepatocellular carcinoma[J]. Vaccines (Basel),2020,8(3):447. doi: 10.3390/vaccines8030447 [7] Wang S,Wang X,Zhou X,et al. DC-CIK as a widely applicable cancer immunotherapy[J]. Expert Opin Biol Ther,2020,20(6):601-607. doi: 10.1080/14712598.2020.1728250 [8] Zhou Z,Qin H,Weng L,Ni Y. Clinical efficacy of DC-CIK combined with sorafenib in the treatment of advanced hepatocellular carcinoma[J]. J BUON,2019,24(2):615-621. [9] Kurebayashi Y,Ojima H,Tsujikawa H,et al. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification[J]. Hepatology,2018,68(3):1025-1041. doi: 10.1002/hep.29904 [10] Di Bella L M,Alampi R,Biundo F,et al. Copper chelation andinterleukin-6 proinflammatory cytokine effects on expression of different proteins involved in iron metabolism in HepG2 cell line[J]. BMC Biochem,2017,18(1):1. doi: 10.1186/s12858-017-0076-2 [11] Li Changfei,Deng Mengmeng,Hu Jun,et al. Chronic inflammation contributes to the development of hepatocellular carcinoma by decreasing miR-122 levels[J]. Oncotarget,2016,7(13):17021-17034. doi: 10.18632/oncotarget.7740 [12] Dai W,Wang Y,Yang T,et al. Down regulation of exosomal CLEC3B in hepatocellular carcinoma promotes metastasis and angiogenesis via AMPK and VEGF signals[J]. Cell Commun Signal,2019,17(1):113. doi: 10.1186/s12964-019-0423-6 [13] Yao Y,Wang T,Liu Y,et al. Co-delivery of sorafenib and VEGF-siRNA via pH-sensitive liposomes for the synergistic treatment of hepatocellular carcinoma[J]. Artif Cells Nanomed Biotechnol,2019,47(1):1374-1383. doi: 10.1080/21691401.2019.1596943 [14] 唐诗聪,陈东,郭瑢,等. miRNA-21调控HER-2阳性乳腺癌的血管生成[J].昆明医科大学学报,2020,41(8):39-45. [15] 雷喜锋,侯峰强,杨少华,等. miR-373在人肝细胞癌中的表达及其作用[J].昆明医科大学学报,2018,39(12):49-53. doi: 10.3969/j.issn.1003-4706.2018.12.010 [16] Sumer S,Aktug Demir N,Kölgelier S,et al. The clinical significance of serum apoptotic cytokeratin 18 neoepitope M30 (CK-18 M30) and matrix metalloproteinase 2 (MMP-2) levels in chronic hepatitis B patients with cirrhosis[J]. Hepat Mon,2013,13(6):e10106. [17] Boukhedouni N,Martins C,Darrigade A S,et al. Type-1 cytokines regulate MMP-9 production and E-cadherin disruption to promote melanocyte loss in vitiligo[J]. JCI Insight,2020,5(11):e133772. [18] 刘坤,朱颖,董蕙,等. 老年晚期肺癌高凝状态患者外周血VEGF、MMP-9的表达[J].昆明医科大学学报,2017,38(1):46-50. doi: 10.3969/j.issn.1003-4706.2017.01.010 -






 下载:
下载: