Role of Phospholipase C epsilon 1 in Neuropathic Pain of Rats with Type 1 Diabetes
-
摘要:
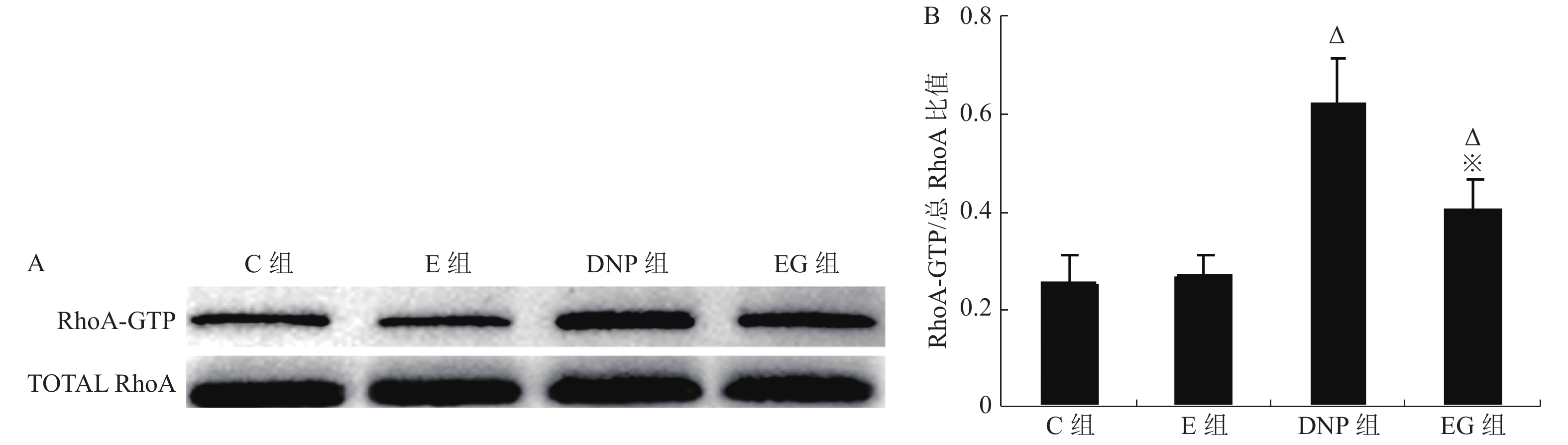
目的 观察糖尿病病理性神经痛(DNP)大鼠脊髓磷脂酶Cε1(PLCE1)表达变化及RhoA抑制剂C3胞外酶对其活性影响,阐明PLCE1在DNP发生中的作用。 方法 24只8周龄、健康雄性SD大鼠随机分为4组(n = 6):正常对照组(C组),单纯RhoA抑制剂组(E组),1型糖尿病DNP模型组(DNP组)和RhoA抑制剂干预组(EG组);静脉注射1%链脲佐菌素(STZ 65 mg/kg)用于建立1型糖尿病DNP大鼠模型。对E组和EG组大鼠进行每天1次为期1周的RhoA抑制剂C3胞外酶(10 pg/10 µL)鞘内注射治疗;C组和DNP组在相应时间点鞘内给予同等容积的溶剂;应用葡萄糖测定仪测定空腹血糖浓度(mmol/L);采用von Frey细丝法和冷热板测痛仪测定大鼠后爪机械刺激缩足反射阈值(MWT)和热刺激缩足反射潜伏期(TWL)作为痛阈观察指标;Western-blot检测脊髓活化RhoA蛋白(RhoA-GTP)、总RhoA蛋白和PLCE1蛋白表达,用脊髓PLCE1蛋白表达水平和RhoA-GTP/总RhoA比值分别评价PLCE1和RhoA活性。ELISA检测脊髓TNF-α和IL-6含量以评价炎症反应。 结果 单纯应用RhoA抑制剂C3胞外酶对非DNP大鼠各项检测指标无明显影响;1型糖尿病DNP大鼠脊髓组织RhoA和PLCE1活性均显著增加(P < 0.05),TNF-α和IL-6含量明显增多,差异有统计学意义(P < 0.05),TWL及MWT显著降低,差异有统计学意义(P < 0.05); C3胞外酶治疗在显著抑制1型糖尿病DNP大鼠脊髓组织RhoA 表达(P < 0.05)的同时,伴有脊髓PLCE1活性的显著降低,差异有统计学意义(P < 0.05)、炎症因子TNF-α和IL-6生成的明显减少,差异有统计学意义(P < 0.05),并显著缓解1型糖尿病DNP大鼠的机械痛敏和热痛敏。 结论 PLCE1在1型糖尿病大鼠DNP中发挥重要作用,其活性受RhoA调控。 Abstract:Objective To elucidate the pathogenic role of phospholipase C epsilon 1 (PLCE1) in neuropathic pain of rats with type 1 diabetes by observing the effect of intrathecal injection of exoenzyme C3 (a RhoA inhibitor) on the activity of PLCE1 and inflammatory response in the spinal cord. Methods Twenty-four 8-week-old healthy male Sprague-Dawley (SD) rats were randomly allocated to healthy rats plus vehicle group (Group C), healthy rats plus exoenzyme C3 group (Group E), type 1 diabetic neuropathic pain rats induced by streptozotocin (STZ) plus vehicle group (Group DNP) and type 1 diabetic neuropathic pain rats plus exoenzyme C3 group (Group EG). The diabetic neuropathic pain model of STZ -induced type 1 diabetes in rats was established by a single intravenous injection of STZ (65 mg/kg). In Group E and Group EG, 10 µL exoenzyme C3 (1 pg/µL ) was injected intrathecally once a day for 7 consecutive days, whereas vehicle was injected intrathecally in Group C and Group DNP. Accu-Chek Compact Plus glucose meter was used to measure fasting blood glucose concentration (mmol/L) in the tail vein once weekly. Western blot was used to detect the expressions of total RhoA and PLCE1 in the spinal cord in rats, while the RhoA activity detection kit was used to measure the expression of RhoA-GTP protein. The expression of PLCE1 protein and the ratio of RhoA-GTP/ total RhoA were used to estimate the activities of PLCE1 and RhoA, respectively. Enzyme-linked immunosorbent assay (ELISA) was used to measure the contents of TNF-α and IL-6 in spinal cord to evaluate proinfammatory cytokines- induced the inflammation. The severity of DNP was evaluated by thermal withdrawal latency (TWL) and mechanical withdrawal threshold (MWT). Results No significant difference was detected between group C and group E. Rats with DNP had a decreased pain threshold (P < 0.05), up-regulated activities of RhoA and PLCE1 (P < 0.05) and increased production of TNF-α and IL-6 in spinal cord (P < 0.05) as compared to the group C and group E. Intrathecal injection of exoenzyme C3 rather than vehicle decreased the activity of RhoA in spinal cord (P < 0.05) of type 1 DNP rats, accompanied by a down-regulation of PLCE1 activity and proinflammatory mediators (P < 0.05) and a relief of DNP (P < 0.05). Conclusion PLCE1 plays an important role in type 1 diabetic neuropathic pain by promoting spinal cord inflammation, and its activity is regulated by RhoA. -
Key words:
- Diabetic neuropathic pain /
- Phospholipase C epsilon 1 /
- RhoA /
- Exoenzyme C3 /
- Inflammation
-
表 1 各组大鼠不同时间点血糖比较[mmol/L,(
$\bar x \pm s$ )]Table 1. Blood glucose concentration of rats in different groups before and after injection of STZ [mmol/L,(
$\bar x \pm s$ )]组别 n T0 T1 T2 T3 C组 6 3.8 ± 0.3 3.5 ± 0.2 3.6 ± 0.3 3.7 ± 0.6 E组 6 3.7 ± 0.2 3.7 ± 0.4 3.8 ± 0.2 3.6 ± 0.5 DNP组 6 3.7 ± 0.4 22.5 ± 1.8∆# 21.2 ± 2.1∆# 24.1 ± 2.4∆# EG组 6 3.6 ± 0.4 21.4 ± 1.7∆# 19.9 ± 1.2∆# 23.9 ± 2.5∆# C组:正常对照;E组:正常对照 + RhoA抑制剂;DNP组:DNP模型; EG组:DNP模型 + RhoA抑制剂; T0:模型建立前;T1:STZ注射后第8天;T2:STZ注射后第36天;T3:RhoA抑制剂注射后第8天;与C组和E组比较,∆P < 0.05;与 T0 比较,#P < 0.05。 表 2 各组大鼠不同时间的TWL和MWT痛阈指标变化(
$\bar x \pm s$ )Table 2. The thermal withdrawal latency (TWL)and the mechanical withdrawal threshold (MWT)in different groups at different time(
$\bar x \pm s$ )组别 TWL(s) MWT(g) n T0 T1 T2 T3 C组 TWL 6 15.8 ± 2.0 15.2 ± 1.4 15.3 ± 1.8 15.7 ± 1.6 MWT 6 14.2 ± 0.7 14.1 ± 0.4 13.8 ± 0.6 14.2 ± 0.5 E组 TWL 6 15.7 ± 1.2 15.0 ± 1.4 15.1 ± 0.9 15.1 ± 1.5 MWT 6 14.2 ± 0.8 14.0 ± 0.8 14.1 ± 0.8 14.0 ± 0.7 DNP组 TWL 6 15.4 ± 1.8 6.5 ± 0.8∆# 9.2 ± 1.1∆# 8.9 ± 1.4∆# MWT 6 14.1 ± 0.5 5.2 ± 0.5∆# 7.8 ± 1.0∆# 7.9 ± 0.9∆# EG组 TWL 6 15.6 ± 1.5 6.7 ± 1.0∆# 9.4 ± 1.1∆# 14.9 ± 1.2▲ MWT 6 14.4 ± 0.4 5.4 ± 0.7∆# 7.9 ± 0.7∆# 13.9 ± 0.5▲ TWL:热刺激缩足反射潜伏期 ;MWT:机械刺激缩足反射阈值 ;T0:模型建立前;T1:STZ注射后第8天;T2:STZ注射后第36天;T3:RhoA抑制剂注射后第8天;与C组和E组比较,∆P < 0.05;与DNP组比较,▲P < 0.05;与 T0 比较,#P < 0.05。 表 3 各组大鼠脊髓组织TNF-α和IL-6含量变化(
$\bar x \pm s$ )Table 3. The changes of TNF-α and IL-6 contents in spinal cordtissues at different groups(
$\bar x \pm s$ )组 别 n TNF-α(ng/mg) IL-6(ng/mg) C组 6 36.7 ± 8.3 23.0 ± 3.4 E组 6 34.2 ± 6.8 22.9 ± 4.7 DNP组 6 75.3 ± 8.8∆ 51.2 ± 7.5∆ EG组 6 52.4 ± 8.3∆▲ 37.2 ± 4.6∆▲ 与C组和E组比较,∆P < 0.05;与DNP组比较,▲P < 0.05;测定时间:STZ注射后第47天。 -
[1] Fitri A,Sjahrir H,Bachtiar A,et al. Modulation of interleukin-8 production by vitamin D supplementation in indonesian patients with diabetic polyneuropathy:A randomized clinical trial[J]. Oman Med J,2020,35(5):e168. doi: 10.5001/omj.2020.110 [2] Kanoko Umezawa,Tatsuya Nagano,Kazuyuki Kobayashi,et al. Phospholipase cε plays a crucial role in neutrophilic inflammation accompanying acute lung injury through augmentation of cxc chemokine production from alveolar epithelial cells[J]. Respir Res,2019,20(1):9. doi: 10.1186/s12931-019-0975-4 [3] Stephanie S Dusaban,Nicole H Purcell,Edward Rockenstein,et al. Phospholipase c epsilon links g protein-coupled receptor activation to inflammatory astrocytic responses[J]. Proc Natl Acad Sci U S A,2013,110(9):3609-3614. doi: 10.1073/pnas.1217355110 [4] Alan V Smrcka,Joan Heller Brown,George G Holz. Role of phospholipase cε in physiological phosphoinositide signaling networks[J]. Cell Signal,2012,24(6):1333-1343. doi: 10.1016/j.cellsig.2012.01.009 [5] Wang C,Song S,Zhang Y,et al. Inhibition of the rho/rho kinase pathway prevents lipopolysaccharide-induced hyperalgesia and the release of TNF-α and IL-1β in the mouse spinal cord[J]. Sci Rep,2015,5:14553. doi: 10.1038/srep14553 [6] Dan Zhu,Tingting Fan,Xinyue Huo,et al. Progressive increase of inflammatory cxcr4 and tnf-alpha in the dorsal root ganglia and spinal cord maintains peripheral and central sensitization to diabetic neuropathic pain in rats[J]. Mediators Inflamm,2019,19:4856156. [7] Dang J K,Wu Y,Cao H,et al. Establishment of a rat model of type II diabetic neuropathic pain[J]. Pain Med,2014,15(4):637-46. [8] Masahiro Ohsawa,Megumi Aasato,Shun-Suke Hayashi,et al. Rhoa/rho kinase pathway contributes to the pathogenesis of thermal hyperalgesia in diabetic mice[J]. Pain,2011,152(1):114-122. doi: 10.1016/j.pain.2010.10.005 [9] 袁凌跃,李江,杨泳,等. 白三烯B4在“肺保护性”通气致肺损伤中的作用机制[J]. 南方医科大学学报,2020,40(10):1465-1471. [10] Stino A M,Smith A G. Peripheral neuropathy in prediabetes and the metabolic syndrome[J]. J Diabetes Investig,2017,8(5):646-655. doi: 10.1111/jdi.12650 [11] Gareth S D Purvis,Massimo Collino,Rodrigo A Loiola,et al. Identification of annexina1 as an endogenous regulator of rhoa,and its role in the pathophysiology and experimental therapy of type-2 diabetes[J]. Front Immunol,2019,10:571. doi: 10.3389/fimmu.2019.00571 [12] Xie Y,Song T,Huo M,et al. Fasudil alleviates hepatic fibrosis in type 1 diabetic rats:involvement of the inflammation and rhoa/rock pathway[J]. Eur Rev Med Pharmacol Sci,2018,22(17):5665-5677. [13] Gao F,Zheng Z M. Animal models of diabetic neuropathic pain[J]. Exp Clin Endocrinol Diabetes,2014,122(2):100-106. doi: 10.1055/s-0033-1363234 [14] Jun-Min Zhong,Yue-Cheng Lu,Jing Zhang. Dexmedetomidine reduces diabetic neuropathy pain in rats through the wnt 10a/β-catenin signaling pathway[J]. Biomed Res Int,2018,18:9043628. [15] Ji-Yuan Zhao,Li Yang,Hu-Hu Bai,et al. Inhibition of protein tyrosine phosphatase 1b in spinal cord dorsal horn of rats attenuated diabetic neuropathic pain[J]. Eur J Pharmacol,2018,827:189-197. doi: 10.1016/j.ejphar.2018.03.012 [16] Lu Y,Lin B,Zhong J. The therapeutic effect of dexmedetomidine on rat diabetic neuropathy pain and the mechanism[J]. Biol Pharm Bull,2017,40(9):1432-1438. doi: 10.1248/bpb.b17-00224 [17] Dolores Piniella,Elena Martínez-Blanco,Ignacio Ibáñez,et al. Identification of novel regulatory partners of the glutamate transporter GLT-1[J]. Glia,2018,66(12):2737-2755. doi: 10.1002/glia.23524 [18] Pyne S,Adams D R,Pyne N J. Sphingosine 1-phosphate and sphingosine kinases in health and disease:Recent advances[J]. Prog Lipid Res,2016,62:93-106. doi: 10.1016/j.plipres.2016.03.001 -






 下载:
下载: