Reductive Amination of Genipin with NaBH3CN to Synthesize Alkaloid-likes and Bioactivity
-
摘要:
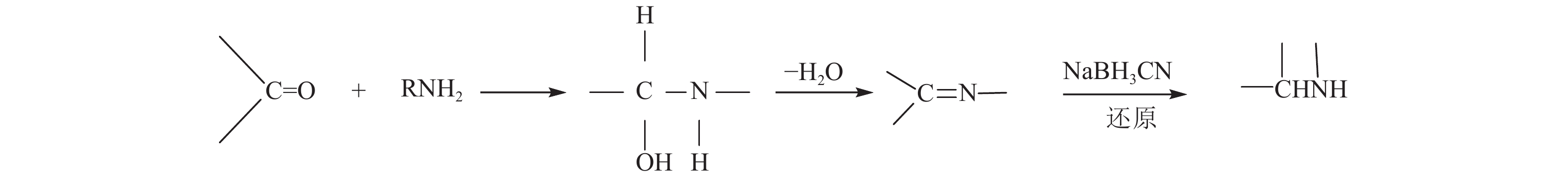
目的 以京尼平苷为原料通过还原胺化反应合成拟生物碱的方法。 方法 京尼平与胺类化合物在氰基硼氢化钠存在下进行还原反应:京尼平与芳基乙胺的甲醇溶液混合后,加入过量氰基硼氢化钠,放置室温下反应3d,产物经石油醚-异丙醇-二乙胺,石油醚-乙酸乙酯等洗脱分离。 结果 合成共得到9个拟生物碱并对部分拟生物碱进行活性筛选,找到治疗2型糖尿病的PTP1B抑制剂。 结论 部分受试化合物对PTP1B有抑制作用。一系列活性衍生物的获得为化合物结构及其生物活性间的构效关系研究打下了基础,有利于寻找具有更高活性的PTP1B抑制剂。 Abstract:Objective To explore a method for the synthesis of alkaloid-likes from Genipin by reductive amination is reported. Methods The reduction of Genipin and amines in the presence of sodium cyanoborohydride: after the methanol solution of Genipin and arylethylamine was mixed, excessive sodium cyanoborohydride was added and the reaction was kept at room temperature for 3 days. The product was eluted and separated on silica gel by petroleum ether-isopropyl alcohol-diethylamine and petroleum ether-ethyl acetate. Results Nine alkaloid-likes were synthesized. Some alkaloid-likes were screened for inhibition activity of PTP1B enzyme for Ⅱ diabetes treatment. Conclusions All of the tested compounds have a certain inhibitory effect on PTP1B. The acquisition of a series of active derivatives has laid a foundation for the study of the structure-activity relationship between the compounds and their bioactivities, so as to facilitate the search for more active PTP1B inhibitors. -
Key words:
- Genipin /
- Alkaloid-likes /
- Reductive amination /
- Inhibition activity against PTP1B
-
表 1 反应产物
Table 1. Products of reaction
R1 R2 C4-C11 1 OCH3 OCH3 α型 2 OCH3 OCH3 β型 3(△3,4) OCH3 OCH3 4(△3,4) OCH3 OH 5 OCH3 OH α型 6 H N(CH3)2 β型 7 H N(CH3)2 α型 8(△3,4) H N(CH3)2 9 H OH β型 10 H OH α型 表 2 9个拟单萜生物碱的13C NMR数据
Table 2. 13C NMR data of 9 monoterpenoid alkaloids
C genipin[7] 1 2 3 4 5 6 7 8 9 1 97.4 50.5 53.7 47.7 55.8 51.7 50.6 47.4 54.3 51.3 3 144.9 55.8 54.5 144.8 50.3 53.4 56.2 146.1 55.4 56.7 4 108.7 43.3 45.7 128.8 42.9 45.2 41.4 98.4 45.4 43.7 5 19.4 39.1 40.4 35.9 38.8 32.0 39.2 39.1 40.2 40.1 6 31.2 30.0 36.1 39.4 30.1 35.9 29.7 34.5 36.6 30.0 7 120.4 125.4 128.6 128.0 125.9 127.8 125.3 126.4 126.5 124.1 8 147.3 147.6 147.3 147.7 146.6 144.9 147.7 144.9 147.0 149.7 9 47.3 42.0 43.5 41.3 41.3 43.5 42.1 41.8 44.8 42.5 10 68.6 60.7 60.4 57.5 61.0 60.7 61.1 60.4 62.0 61.5 11 165 173.8 174.6 169.4 173.2 174.7 173.9 170.0 176.2 174.2 酯OCH3 50.8 51.5 51.8 50.5 51.6 54.4 51.4 57.7 52.3 51.5 1′ 61.1 60.5 61.3 60.4 60.0 60.9 50.3 60.6 60.5 2′ 33.1 32.9 35.3 32.5 29.2 30.1 35.7 33.3 33.0 3′ 132.6 132.3 130.9 131.0 128.0 128.2 129.2 132.1 131.5 4′ 111.2 111.2 111.4 111.4 129.1 129.3 126.8 130.6 130.3 5′ 148.9 148.8 148.9 147.3 113.1 113.0 113.1 116.2 115.9 6′ 147.3 145.0 145.7 144.2 149.2 149.2 149.4 156.6 156.5 7′ 112.0 111.9 112.0 114.4 113.1 113.0 113.1 116.2 116.2 8′ 120.5 120.4 120.7 121.3 129.1 129.3 126.8 130.6 130.6 芳OCH N(CH3)2 55.9 55.2 55.9 55.8 55.9 55.7 55.9 40.8 40.9 40.7 表 3 部分化合物对PTP1B的抑制率( $\bar x \pm s $)
Table 3. The inhibition activity of part of products against PTP1B ( $\bar x \pm s $)
试样 试样浓度 抑制率(%) 2 20 μg/mL 16.50 ± 1.79 9 20 μg/mL 15.13 ± 4.51 注:抗PTP1B活性筛选由国家新药筛选中心完成。 -
[1] 国家医药管理局中草药情报中心站. 植物药有效成分手册[M]. 北京: 人民卫生出版社, 1986: 490-491. [2] Gord W A. Convenient synthesis of 1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizine[J]. J Org Chem,1972,37(11):1833-1835. doi: 10.1021/jo00976a039 [3] Shan M, Yu S, Yan H, et al. A review on the phytochemistry, pharmacology pharmacokinetics and toxicology of geniposide, a natural product[J]. Molecules, 217, 22(10): 1-29. [4] 王娟娟,秦雪梅,高晓霞,等. 杜仲化学成分、药理活性和质量控制现状研究进展[J]. 中草药,2017,48(15):3228-3237. [5] 白涛,任乐乐,刘萌萌,等. 京尼平苷对高糖高脂诱导的岛β细胞胰岛素分泌的影响[J]. 中药物与临床,2019,19(12):1949-1950. [6] 那莎等. 栀子及其有效成分的药理研究进展[J]. 研究与展望,2005,12(1):90. [7] 杨全军,范明松,孙兆林,等. 栀子化学成分、药理作用及体内过程研究进展[J]. 中国现代中药,2010,12(9):7-12. doi: 10.3969/j.issn.1673-4890.2010.09.002 [8] 王菲菲,张聿梅,郑笑为,等. 环烯醚萜类化合物的结构和生物学活性研究进展[J]. 中国药事,2019,33(3):323-330. [9] 姚新生. 天然产物化学[M]. 第2版. 北京: 人民卫生出版社, 1999: 439. [10] Leonard J. Recent progress in the chemistry of monoterpenoid indole alkaloid derined from s ecologanin[J]. Nat Prod Rep,1999,16(21):319-338. [11] Yu Yang,Xie Zuo-lei,Gao Hao,et al. Bioactive iridoid glucosides from the fruit of Gardenia jasminoides[J]. J Nat Prod,2009,72(12):1459-1464. [12] Hiro Iets S,Hih T. Zwei neue iridoidglucoside aus Gardeniajasminoides:gardenosid und geniposid[J]. Tetrahedron Letters,1969,10(28):2347-2350. doi: 10.1016/S0040-4039(01)88161-2 [13] Rich F B,Mark D B,H. D D. The Cyanohydridoborate Anion as a Selective Reducing Agent[J]. J Amer Chem Soc,1971,93(12):2897-2904. doi: 10.1021/ja00741a013 [14] Wang X Y,Bergdahl K,Heijbel A,et al. Analysis of in vitro interactions of protein tyrosine phosphatase 1B with insulin receptors[J]. Molecular and Cellular Endocrinolog,2001,173(1-2):109-119. doi: 10.1016/S0303-7207(00)00402-0 [15] Klaman L D,Boss O,Peroni O D,et al. Increasedenergy expenditure,decreased adiposity,and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice[J]. Molecular and Cellular Biolog,2000,20(15):5479-5489. doi: 10.1128/MCB.20.15.5479-5489.2000 [16] Haque A,Andersen Jannik N,Salmeen A,et al. Conformation-sensing antibodies stabilize the oxidized form of PTP1B and inhibit its phosphatase activity[J]. Cell,2011,147(1):185-198. doi: 10.1016/j.cell.2011.08.036 [17] Li H,Tang G H,Zhang Y,et al. A new carotane sesquiterpene from Walsura robusta[J]. Chinese Journal of Natural Medicine,2013,11(1):84-86. doi: 10.1016/S1875-5364(13)60014-X -






 下载:
下载: