Influence of DC-Chol Cationic Liposome Adjuvant on the Immune Effect of Tetravalent Influenza Vaccine
-
摘要:
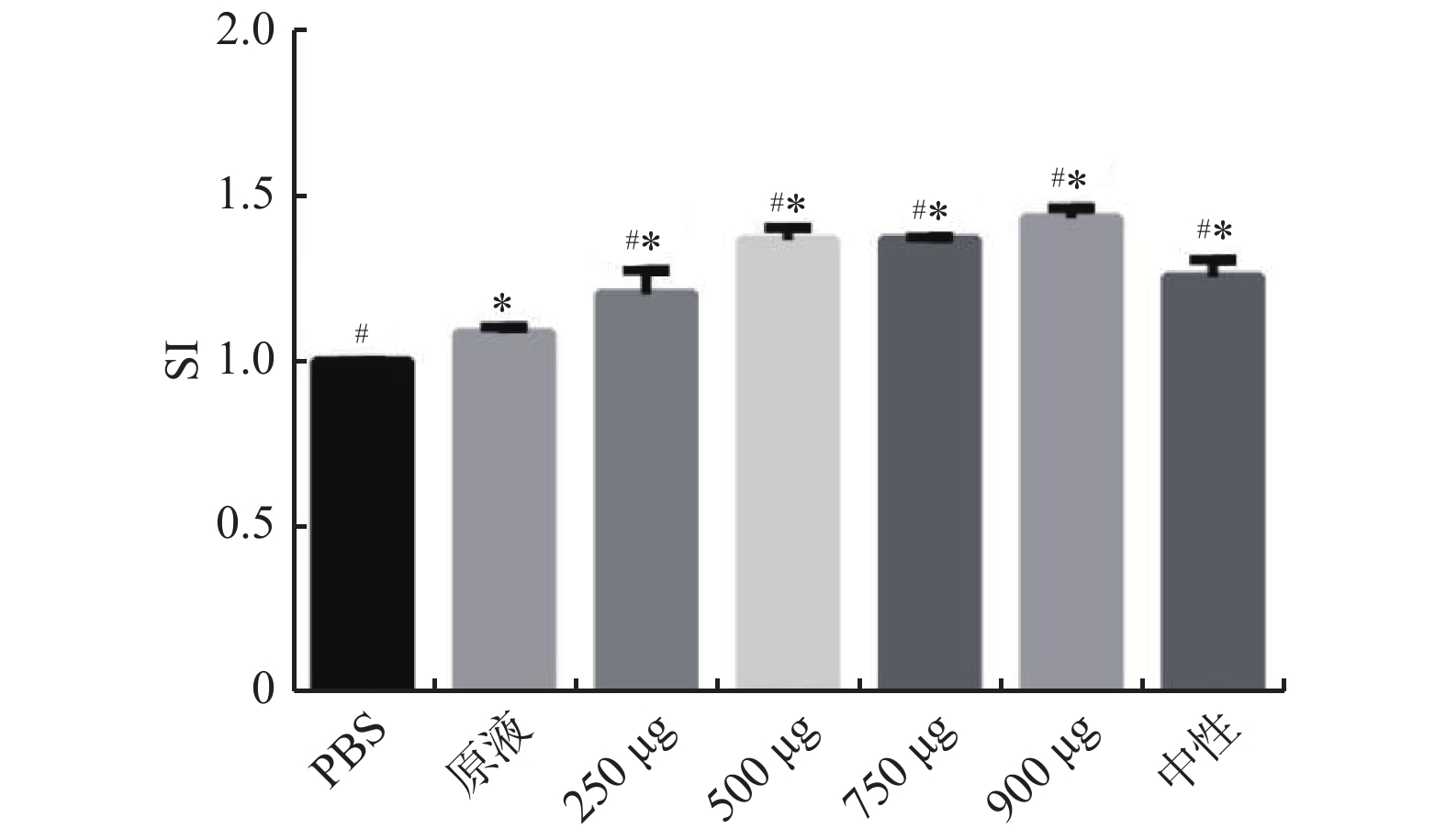
目的 观察阳离子脂质体DC-Chol修饰的四价流感疫苗的免疫原性。 方法 采用薄膜分散结合冻融-冻干法制备DC-Chol脂质体,检测其包封率。将小鼠分为七组,为DC-Chol脂质体疫苗组(250 µg/只、500 µg/只、750 µg/只、900 µg/只)、PBS组、中性脂质体组、疫苗原液组(n = 3),通过MTT法确定DC-Chol脂质体的最佳剂量。将小鼠分为四组,DC-Chol脂质体组、疫苗原液组、中性脂质体组以及PBS对照组,腹腔免疫小鼠,于第7天、14天、28天处死,MTT法和T淋巴细胞表面标记实验检测小鼠淋巴细胞增殖情况观察免疫原性。 结果 MTT实验表明,DC-Chol修饰脂质体的最佳用量为500 µg/鼠(P < 0.05);T淋巴细胞表面标记实验显示,DC-Chol 阳离子脂质体能明显增强脾淋巴细胞数(P < 0.05)。 结论 与中性脂质体相比,DC-Chol 阳离子脂质体作为四价流感疫苗的佐剂具有良好的免疫增强作用。 Abstract:Objective To study the immunogenicity of the tetravalent influenza vaccine modified by cationic liposome DC-Chol. Methods DC-Chol liposomes were prepared by thin film dispersion-freeze-thaw- freeze-drying method, and the encapsulation rate was determined. The mice were divided into seven groups, DC-Chol liposome vaccine group (250 µg/mouse, 500 µg/mouse, 750 µg/mouse, 900 µg/mouse), PBS group, vaccine stock solution group, neutral liposome group (n = 3)Determine the optimal dose of DC-Chol liposomes by MTT method. The mice were divided into 4 groups, DC-Chol liposome group, vaccine stock solution group, neutral liposome group and PBS control group. Mice were immunized through the peritoneum and sacrificed on the 7, 14, and 28 days. The proliferation of mouse lymphocytes was detected by MTT method and T lymphocyte surface labeling experiment to study its immunogenicity. Results The MTT experiment showed that the optimal dose of DC-Chol modified liposomes was 500 μg/rat (P < 0.05); T lymphocyte surface labeling experiments showed that DC-Chol cationic liposomes significantly increased the number of splenic lymphocytes (P < 0.05). Conclusion Compared with neutral liposomes, DC-Chol cationic liposomes as an adjuvant of tetrovalent influenza vaccine has good immune enhancement effect. -
Key words:
- Liposome /
- DC-Chol /
- Influenza vaccine /
- Cellular immunity
-
表 1 不同含量的DC-Chol阳离子脂质体的包封率
Table 1. Encapsulation efficiency of DC-Chol cationic liposomes with different contents
DC-Chol含量(µg/鼠) 包封率(%) 250 59.17 500 70.44 750 68.78 900 68.78 -
[1] Okuda K,Xin K Q,Haruki A,et al. Transplacental genetic immunization after intravenous delivery of plasmid DNA to pregnant mice[J]. Journal of Immunology,2001,167(9):5478-5484. doi: 10.4049/jimmunol.167.9.5478 [2] Dennis Christensen,Korsholm Karen Smith,Andersen Peter,et al. Cationic liposomes as vaccine adjuvants[J]. Expert Review of Vaccines,2011,10(4):513-521. doi: 10.1586/erv.11.17 [3] Inoh Y,Tadokoro S,Tanabe H,et al. Inhibitory effects of a cationic liposome on allergic reaction mediated by mast cell activation[J]. Biochemical Pharmacology,2013,86(12):1731-1738. doi: 10.1016/j.bcp.2013.09.023 [4] Henriksen-Lacey M,Christensen D,Bramwell V W,et a1. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response[J]. J Control Release,2010,145(2):102-108. doi: 10.1016/j.jconrel.2010.03.027 [5] 肖勇翔,陈志祥,陆伟根. 阳离子脂质体疫苗佐剂研究进展[J]. 世界临床药物,2013,34(1):41-48. [6] Ciani L,Ristori S,Salvati A,et al. DOTAP/DOPE and DC-Chol/DOPE lipoplexes for gene delivery:zeta potential measurements andelectron spin resonance spectra[J]. Biochim Biophys Acta,2004,1664(1):70-79. doi: 10.1016/j.bbamem.2004.04.003 [7] Colosimo A,Serafino A,Sangiuolo F,et al. Gene transfection efficiency of tracheal epithelial cells by DC-chol-DOPE/DNA complexes[J]. Biochim Biophys Acta,1999,1419(2):186-194. doi: 10.1016/S0005-2736(99)00067-X [8] Lendemans D G,Myschik J,Hook S,et al. Cationic cage-like complexes formed by DC-cholesterol,Quil-A,and phospholipid[J]. Journal of Pharmaceutical Ences,2010,94(8):1794-1807. [9] Myschik Julia,Warren T Mcburney,Thomas Rades. Immunostimulatory lipid implants containing Quil-A and DC-cholesterol[J]. International Journal of Pharmaceutics,2008,363(1):91-98. [10] Gao Jie,Ochyl Lukasz J,Yang Ellen,et al. Cationic liposomes promote antigen cross-presentation in dendritic cells by alkalizing the lysosomal pH and limiting the degradation of antigens.[J]. International Journal of Nanomedicine,2017,12(10):1251-1264. [11] Mcburney Warren T,Dirk G Lendemans,Myschik Julia. In vivo activity of cationic immune stimulating complexes(PLUSCOMs)[J]. Vaccine,2008,26(35):4549-4556. doi: 10.1016/j.vaccine.2008.06.024 [12] 乔建斌. 冻融-冻干法制备流感疫苗脂质体及其细胞免疫研究[D]. 昆明: 昆明医科大学硕士学位论文, 2014. [13] 刘洁,马波,鲁卫东,等. 单价流感疫苗脂质体干粉细胞免疫研究[J]. 南京工业大学学报(自然科学版),2011,33(06):102-106. doi: 10.3969/j.issn.1671-7627.2011.06.021 [14] Yifan Ma,Zhuang Yan,Xie Xiaofang,et al. The role of surface charge density in cationic liposome-promoted dendritic cell maturation and vaccine-induced immune responses[J]. Nanoscale,2011,3(5):2307-2314. doi: 10.1039/c1nr10166h [15] Brunel F,Darbouret A,Ronco J. Cationic lipid DC-Chol induces an improved and balanced immunity able to overcome the unresponsiveness to the hepatitis B vaccine.[J]. Vaccine,1999,17(17):2192-2203. doi: 10.1016/S0264-410X(98)00492-7 [16] Rui T,Hidehiko S,Saeko T,et al. Nasal vaccination with pneumococcal surface protein A in combination with cationic liposomes consisting of DOTAP and DC-chol confers antigen-mediated protective immunity against Streptococcus pneumoniae infections in mice[J]. International Immunopharmacology,2018,61(2):385-393. [17] Jinju L,Jun A H. PEGylated DC-Chol/DOPE cationic liposomes containing KSP siRNA as a systemic siRNA delivery Carrier for ovarian cancer therapy[J]. Biochemical and Biophysical Research Communications,2018,503(3):1-7. [18] Seraj S,Ahn H J,Lee J. Systemic delivery of Eg5 shRNA-expressing plasmids using PEGylated DC-Chol/DOPE cationic liposome:long-term silencing and anticancer effects in vivo[J]. Biochemical Pharmacology,2019,166:192-202. doi: 10.1016/j.bcp.2019.05.021 -






 下载:
下载: