Aqueous Extract of Moringa Oleifera
-
摘要:
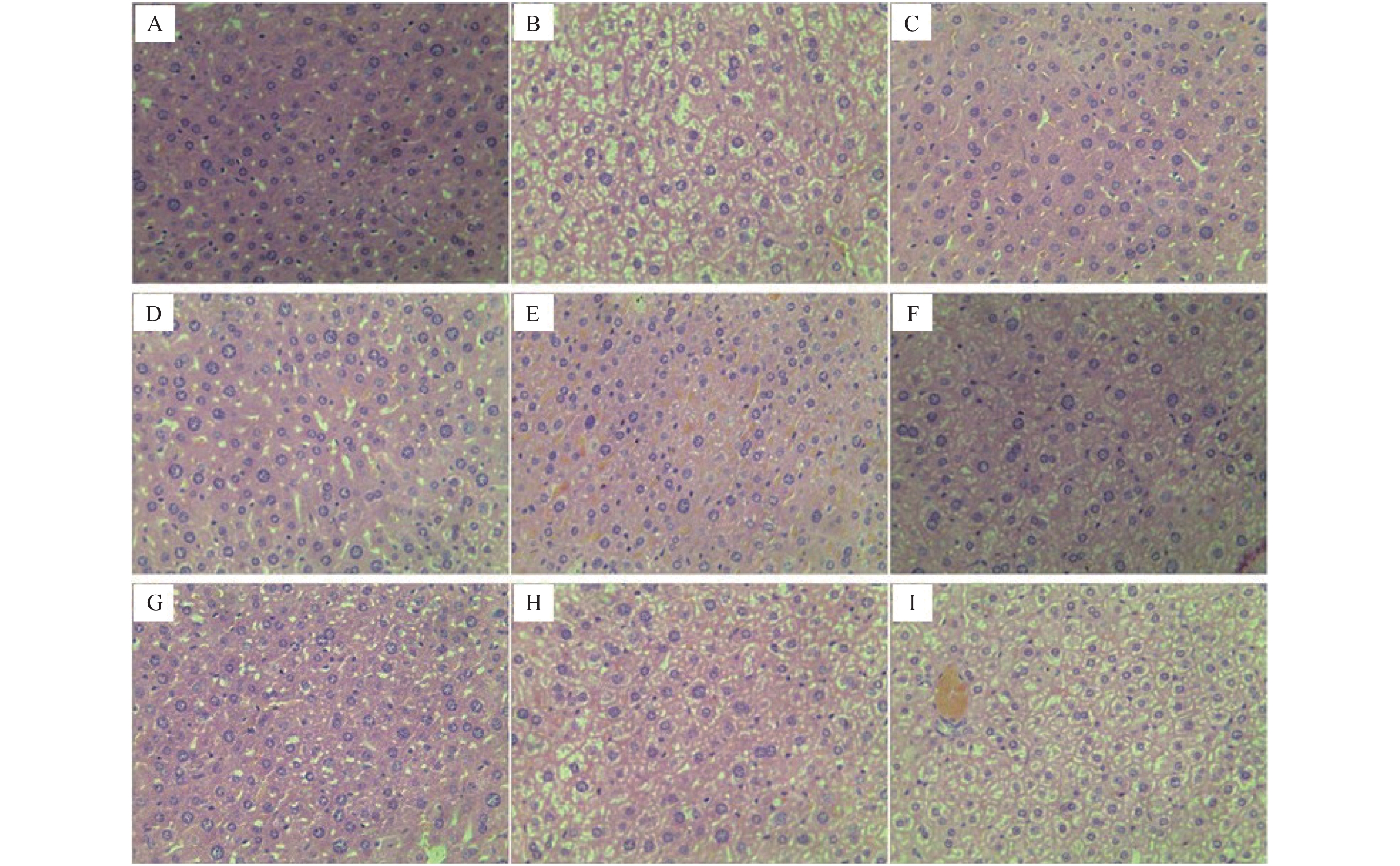
目的 研究辣木叶水提取物(extract of Moringa oleifera leaves,EMO)对奥氮平(olanzapine,OLA)诱导的小鼠代谢紊乱的保护作用和机制。 方法 90只雌性小鼠随机分为对照组、模型组(OLA,3 mg/kg)、阳性药组[二甲双胍(MET) + OLA,75 mg/kg + 3 mg/kg]、EMO组(400、200、100 mg/kg)、OLA + EMO组(OLA + EMO-H/M/L,3 mg/kg + 400、200、100 mg/kg)组。各组灌胃处理14 d,检测小鼠体重增量、进食量、饮水量、空腹血糖浓度(fasting blood-glucose,FBG)、血脂(TCH、TG、HDL-C、LDL-C)含量、血清瘦素、胃饥饿素、丙二醛(malondialdehyde,MDA)含量、血清超氧化物歧化酶(superoxide dismutase,SOD)和谷胱甘肽过氧化物酶(glutathion peroxidase,GSH-Px)活力、肝脏脂肪酸合成酶(fatty acid synthetase,FAS)和脂肪分化相关蛋白(adipose differentiation-related protein,ADRP)基因mRNA表达、肝脏组织病理损伤。 结果 与对照组比较,OLA组摄食量显著增加(P < 0.01),FBG和THC、TG、LDL-C含量显著的升高(P < 0.05或P < 0.01),血清瘦素和胃饥饿素含量显著增加(P < 0.01),肝脏FAS和ADRP基因mRNA表达显著上调(P < 0.05或P < 0.01),血清GSH-Px酶活力显著降低(P < 0.05),MDA含量显著增加(P < 0.05),肝脏组织损伤严重。与OLA组比较,OLA + EMO-H/M组进食、饮水量显著减少(P < 0.05或P < 0.01),体重增量显著降低(P < 0.05或P < 0.01),血清FBG和THC、TG、LDL-C含量显著降低(P < 0.05),瘦素和胃饥饿素含量显著降低(P < 0.05),肝脏FAS和ADRP基因mRNA表达显著下调(P < 0.05),血清SOD和GSH-Px酶活力显著升高(P < 0.05),MDA含量显著降低(P < 0.05),肝脏组织损伤显著改善。EMO组与对照组比较,上述指标差异无统计学意义( P > 0.05)。 结论 辣木叶能够部分逆转奥氮平诱导的糖脂代谢紊乱,其作用机制一方面是辣木叶的抗氧化作用保护组织器官的正常功能,减少瘦素和胃饥饿素的分泌,减轻暴饮暴食症状;另一方面,辣木叶保护肝脏组织细胞功能,下调脂肪酸合成和转运相关基因的表达,降低血脂含量。 Abstract:Objective To explore the protective effect and mechanisms of aqueous extract of Moringa oleifera leaves (EMO) on olanzapine (OLA) induced metabolic disorders in mice. Method 90 female mice were randomly divided into control group, OLA group (3 mg/kg), positive control drug group (OLA + metformin (MET), 3 mg/kg + 75 mg/kg), EMO group (EMO-H/M/L. 400/200/100 mg/kg), OLA + EMO group (OLA + EMO-H/M/L, 3 mg/kg + 400/200/100). Each group was given intragastric administration for 14 days, and weight gain, food and water intake, fasting blood glucose, blood lipid, serum leptin, ghrelin, malondialdehyde (MDA), serum peroxidase dismutase (SOD) activity, glutathione peroxidase (GSH-Px) activity, liver fatty acid synthase (FAS) and adipose differentiation related protein (ADRP) gene mRNA expression, liver tissue pathology damage were detected. Result Compared with control group, in OLA group food intake increased significantly (P < 0.01), the contents of FBG, THC, TG and LDL-C, the levels of serum leptin and ghrelin increased significantly (P < 0.05 or P < 0.01), and the expression of liver FAS and ADRP genes was significantly increased (P < 0.01). Serum SOD and GSH-Px enzyme activities were significantly reduced (P < 0.05), MDA content was significantly increased (P < 0.05), and liver tissue damage was severe. Compared with the OLA group, in OLA + EMO-H / M group food and water intake significantly reduced (P < 0.05 or P < 0.01), body weight gain significantly reduced (P < 0.05 or P < 0.01), serum FBG and THC, TG, and LDL-C levels were significantly reduced (P < 0.05), leptin and ghrelin levels were significantly reduced (P < 0.05). Liver FAS and ADRP gene mRNA expression was significantly down-regulated, serum SOD and GSH-Px enzyme activities were significantly increased (P < 0.05), MDA concentration was significantly reduced (P < 0.05), and liver tissue damage was partially reversed. Compared the Control group, in the EMO group the above indicators were not significantly different. Conclusion EMO may partially reverse the glucose and lipid metabolism disorder induced by olanzapine. The mechanism is that the antioxidant effect protects the normal functions of tissues and organs, reduces the secretion of leptin and ghrelin, and reduces the symptoms of overeating. In addition, it can protect the cellular function of liver tissue, down-regulate the expression of genes related to fatty acid synthesis and transport, and reduce the content of blood lipid. -
Key words:
-
Moringa oleifera leaves / - Olanzapine /
- Metabolic syndrome /
- Antioxidant
-
-
表 1 引物序列
Table 1. Primer sequences
基因名称 正向引物序列(5′−3′) 反向引物序列(5′−3′) FAS GACTCGCTACTGACACGAC CGAGTTGAGCTGGGTTAGGG ADRP GTGTGTGAGATGGCCGAGAA AACAATCTCGGACGTTGGCT β-actin CGTTGATCCGTAAAGACCTC TAGGAGCCTGGGCAGTAATCT 表 2 辣木叶对小鼠进食量、饮水量和体重增长量的影响[(
$ \bar x \pm s$ ),n = 10]Table 2. Effect of EMO on food and water intake,body weight gain in mice [(
$ \bar x \pm s$ ),n = 10]分组 进食量(g) 饮水量(mL) 体重增量(g) 体重增长比(%) 对照组 3.57 ± 0.30 4.42 ± 0.32 6.89 ± 0.45 29.52 ± 2.23 OLA组 4.91 ± 1.03** 4.91 ± 0.44** 10.75 ± 1.22** 46.08 ± 5.58** OLA+MET组 4.05 ± 0.62# 4.52 ± 0.56# 7.26 ± 0.63## 31.65 ± 2.65## EMO-H组 3.43 ± 0.18 4.44 ± 0.31 7.22 ± 0.51 30.89 ± 2.83 EMO-M组 3.51 ± 0.25 4.46 ± 0.32 7.19 ± 0.57 30.83 ± 2.33 EMO-L组 3.34 ± 0.92 4.45 ± 0.33 7.20 ± 0.85 30.86 ± 4.27 OLA+EMO-H组 3.92 ± 0.48## 4.47 ± 0.33## 7.79 ± 0.46## 32.68 ± 2.22## OLA+EMO-M组 4.06 ± 0.47# 4.50 ± 0.30# 9.32 ± 1.22# 40.01 ± 6.14# OLA+EMO-L组 4.25 ± 0.73 4.49 ± 0.29## 9.96 ± 1.40 42.51 ± 7.18 与对照组比较,*P < 0.05,**P < 0.01;与OLA组比较,#P < 0.05,##P < 0.01。 表 3 小鼠血清TCH、TG、HDL-C、LDL-C含量 [(
$ \bar x \pm s$ ),n = 10]Table 3. The concentration of serum THC,TG,HDL-C,LDL-C in mice [(
$ \bar x \pm s$ ),n = 10]分组 TCH(mmol/L) TGmmol/L) HDL-C(mmol/L) LDL-C(mmol/L) 对照组 2.39 ± 0.34 1.49 ± 0.11 2.65 ± 0.26 1.02 ± 0.12 OLA组 3.11 ± 0.59** 2.02 ± 0.24** 2.36 ± 0.27* 1.43 ± 0.20** OLA+MET组 2.41 ± 0.39## 1.66 ± 0.32## 2.42 ± 0.35 1.25 ± 0.16# EMO-H组 2.31 ± 0.50 1.53 ± 0.13 2.69 ± 0.40 1.09 ± 0.12 EMO-M组 2.63 ± 0.40 1.54 ± 0.16 2.75 ± 0.36 1.07 ± 0.08 EMO-L组 2.41 ± 0.37 1.52 ± 0.13 2.73 ± 0.34 1.06 ± 0.12 OLA+EMO-H组 2.55 ± 0.56# 1.70 ± 0.36# 2.48 ± 0.34 1.27 ± 0.11* OLA+EMO-M组 2.60 ± 0.47# 1.72 ± 0.37# 2.50 ± 0.31 1.27 ± 0.12* OLA+EMO-L组 2.75 ± 0.52 1.75 ± 0.22# 2.52 ± 0.31 1.34 ± 0.10 与对照组比较,*P < 0.05,**P < 0.01;与OLA组比较,#P < 0.05,##P < 0.01。 表 4 小鼠空腹血糖和血清瘦素和胃饥饿素含量(
$ \bar x \pm s$ ,n = 10)Table 4. Levels of fasting blood glucose and serum leptin,ghrelin in mice (
$ \bar x \pm s$ ,n = 10)分组 FBG(mmol/L)(第0天) FBG(mmol/L)(第14天) Leptin(ng/mL) Ghrelin(pg/mL) 对照组 4.44 ± 0.11 4.51 ± 0.10 8.23 ± 0.92 152.51 ± 34.27 OLA组 4.42 ± 0.16 6.77 ± 1.93** 13.24 ± 3.19** 242.01 ± 64.53** OLA+MET组 4.42 ± 0.21 4.89 ± 1.26## 12.36 ± 1.52 229.33 ± 45.69 EMO-H组 4.45 ± 0.10 4.48 ± 0.11 8.86 ± 0.91 140.11 ± 24.06 EMO-M组 4.43 ± 0.13 4.53 ± 0.19 8.79 ± 0.60 135.03 ± 27.22 EMO-L组 4.41 ± 0.15 4.42 ± 0.15 8.47 ± 0.95 144.41 ± 24.69 OLA+EMO-H组 4.44 ± 0.12 5.71 ± 0.87# 10.85 ± 1.16# 186.82 ± 33.59# OLA+EMO-M组 4.37 ± 0.11 5.91 ± 0.39# 10.98 ± 1.10# 190.15 ± 37.26# OLA+EMO-L组 4.43 ± 0.10 6.62 ± 1.68# 12.10 ± 1.62 226.51 ± 42.43 与对照组比较,*P < 0.05,**P < 0.01;与OLA组比较,#P < 0.05,##P < 0.01。 表 5 小鼠血清SOD、GSH-Px活力和MDA含量[(
$ \bar x \pm s$ ),n = 10]Table 5. Enzyme activity of SOD,GSH-Px and concentration of MDA in mice [(
$ \bar x \pm s$ ),n = 10]分组 SOD(U/mL) GSH-Px(U/mL) MDA(μmol/mL) 对照组 175.10 ± 14.48 238.97 ± 30.08 7.96 ± 1.45 OLA组 149.27 ± 24.41* 193.29 ± 16.64** 10.81 ± 8.44* OLA+MET组 176.22 ± 26.55# 224.65 ± 59.26# 8.15 ± 1.66# EMO-H组 158.93 ± 17.13* 208.98 ± 33.46* 6.84 ± 1.58 EMO-M组 169.74 ± 14.49 212.06 ± 23.94* 7.14 ± 1.27 EMO-L组 173.91 ± 9.98 236.89 ± 25.83 7.52 ± 1.55 OLA+EMO-H组 172.72 ± 18.06# 225.96 ± 40.05# 8.20 ± 1.19# OLA+EMO-M组 169.52 ± 15.61# 222.60 ± 37.95# 8.80 ± 1.81 OLA+EMO-L组 165.32 ± 24.16 211.91 ± 34.46 10.03 ± 1.58 与对照组比较,*P < 0.05,**P < 0.01;与OLA组比较,#P < 0.05,##P < 0.01。 表 6 小鼠肝脏FAS、ADRP基因mRNA表达变化[(
$ \bar x \pm s$ ),n = 5]Table 6. FAS and ADRP mRNA expression [(
$ \bar x \pm s$ ),n = 5]分组 FAS ADRP 对照组 1.00 ± 0.08 1.00 ± 0.06 OLA组 1.29 ± 0.20* 1.61 ± 0.26** OLA + MET组 1.06 ± 0.08# 1.30 ± 0.18# EMO-H组 1.05 ± 0.08 1.06 ± 0.06 EMO-M组 1.08 ± 0.07 1.08 ± 0.14 EMO-L组 1.08 ± 0.08 0.98 ± 0.12 OLA + EMO-H组 1.05 ± 0.09# 1.21 ± 0.10# OLA + EMO-M组 1.08 ± 0.05# 1.28 ± 0.17# OLA + EMO-L组 1.18 ± 0.10 1.51 ± 0.17 与对照组比较,*P < 0.05,**P < 0.01;与OLA组比较,#P < 0.05,##P < 0.01。 -
[1] Lieberman J A,Stroup T S,McEvoy J P,et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia[J]. N Engl J Med,2005,353(12):1209-1223. doi: 10.1056/NEJMoa051688 [2] Lieberman J A. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia:efficacy,safety and cost outcomes of CATIE and other trials[J]. J Clin Psychiatry,2007,68(2):4. doi: 10.4088/JCP.0207e04 [3] Brixner D I,Said Q,Corey-Lisle P K,et al. Naturalistic impact of second-generation antipsychotics on weight gain[J]. Ann Pharmacother,2006,40(4):626-632. doi: 10.1345/aph.1G564 [4] Riordan H J,Antonini P,Murphy M F. Atypical antipsychotics and metabolic syndrome in patients with schizophrenia:Risk factors,monitoring,and healthcare implications[J]. Am Health Drug Benefits,2011,5(4):292-302. [5] Kirk S L,Glazebrook J,Grayson B,et al. Olanzapine-induced weight gain in the rat:Role of 5-HT2C and histamine H1 receptors[J]. Psychopharmacology(Berl),2009,207(1):119-125. doi: 10.1007/s00213-009-1639-8 [6] Boyda H N,Procyshyn R M,Tse L,et al. Differential effects of 3 classes of antidiabetic drugs on olanzapine-induced glucose dysregulation and insulin resistance in female rats[J]. J Psychiatry Neurosci,2012,37(6):407-415. doi: 10.1503/jpn.110140 [7] Berkovich L,Earon G,Ron I,et al. Moringa oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells[J]. BMC Complement Altern Med,2013,13(1):212-218. doi: 10.1186/1472-6882-13-212 [8] Gowrishankar R,Kumar M,Menon V,et al. Trace element studies on tinospora cordifolia(Menispermaceae),ocimum sanctum(Lamiaceae),Moringa oleifera(Moringaceae),and phyllanthus niruri(Euphorbiaceae) using PIXE[J]. Biol Trace Elem Res,2010,133(3):357-363. doi: 10.1007/s12011-009-8439-1 [9] Joung H,Kim B,Park H,et al. Fermented Moringa oleifera decreases hepatic adiposity and ameliorates glucose intolerance in high-fat diet-induced obese mice[J]. J Med Food,2017,20(5):439-447. doi: 10.1089/jmf.2016.3860 [10] Yassa H D,Tohamy A F. Extract of Moringa oleifera leaves ameliorates streptozotocin-induced diabetes mellitus in adult rats[J]. Acta Histochem,2014,116(5):844-854. doi: 10.1016/j.acthis.2014.02.002 [11] Jaiswal D,Kumar Rai P,Kumar A,et al. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats[J]. J Ethnopharmacol,2009,123(3):392-397. doi: 10.1016/j.jep.2009.03.036 [12] Stefanidis A,Watt M J,Cowley M A,et al. Prevention of the adverse effects of olanzapine on lipid metabolism with the antiepileptic zonisamide[J]. Neuropharmacology,2017,123(1):55-66. doi: 10.1016/j.neuropharm.2017.04.010 [13] Liu X,Deng C,Cao S,et al. Acute effects of oral olanzapine treatment on the expression of fatty acid and cholesterol metabolism-related gene in rats[J]. Life Sci,2015,128(1):72-78. [14] Liebig M,Gossel M,Pratt J,et al. Profiling of energy metabolism in olanzapine-induced weight gain in rats and its prevention by the CB1-antagonist AVE1625[J]. Obesity(Silver Spring),2012,18(10):1952-1958. [15] Shertzer H G,Kendig E L,Nasrallah H A,et al. Protection from olanzapine-induced metabolic toxicity in mice by acetaminophen and tetrahydroindenoindole[J]. Int J Obes(Lond),2010,6(34):970-979. [16] Chung Shu Yang, Hong Wang, Zachary Paul Sheridan. Studies on prevention of obesity, metabolic syndrome, diabetes, cardiovascular diseases and cancer by tea[J]. Journal of Food and Drug Analysis,2018,26(1):1-13. doi: 10.1016/j.biopha.2017.05.113 [17] Patel J K,Buckley P F,Woolson S,et al. Metabolic profiles of second-generation antipsychotics in early psychosis:Findings from the CAFE study[J]. Schizophrenia Research,2009,111(1-3):9-16. doi: 10.1016/j.schres.2009.03.025 [18] 刘素芳,魏平,王世贵,等. 不同剂量氯氮平与精神分裂症患者血脂、血糖代谢的对照研究[J]. 中华精神科杂志,2005,38(2):82-85. doi: 10.3760/j:issn:1006-7884.2005.02.006 -






 下载:
下载: