Expression of B7-H4 in Intrahepatic Cholangiocarcinoma and Its Clinical Significance
-
摘要:
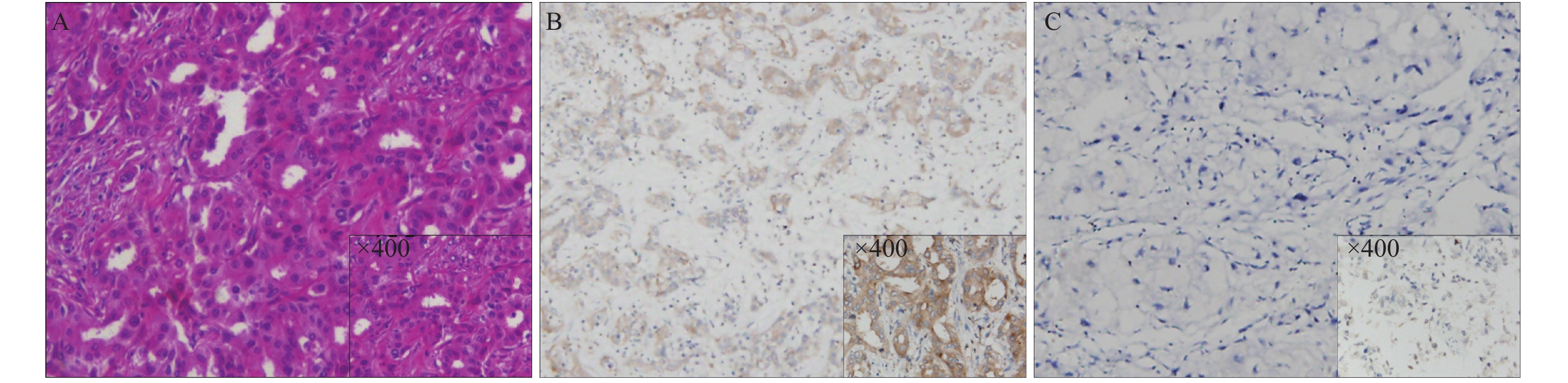
目的 探讨协同共刺激分子B7-H4在肝内胆管癌组织中的表达及其临床意义。 方法 采用免疫组化检测90例肝内胆管癌(intrahepatic cholangiocarcinoma,ICC)及其癌旁组织中B7-H4的表达,并分析B7-H4表达与患者临床病理特征及预后关系。 结果 在肝内胆管癌中B7-H4表达率为44.4%,明显高于癌旁组织,且肝内胆管癌中B7-H4表达上升与TNM分期、肿瘤的分化、淋巴结转移及不良预后相关(P < 0.05)。生存分析提示B7-H4高表达组患者术后总生存率明显低于B7-H4低表达组(P = 0.005)。Cox比例风险回归模型分析表明B7-H4是肝内胆管癌患者的独立预后因子。 结论 B7-H4在肝内胆管癌组织中表达增加,且B7-H4高表达与ICC患者恶性病理特征及不良预后密切相关,对预估患者预后有一定临床指导意义。 -
关键词:
- 协同共刺激分子B7-H4 /
- 肝内胆管癌 /
- 病理 /
- 预后
Abstract:Objective To investigate the expression of costimulatory molecule B7-H4 in intrahepatic cholangiocarcinoma (ICC)and its clinical significance. Methods Immunohistochemistry was used to detect the expression of B7-H4 in 90 cases of intrahepatic cholangiocarcinoma and its adjacent tissues, and analyzed the correlation between B7-H4 expression and the clinicopathological characteristics and prognosis of the patients. Results The expression rate of B7-H4 in intrahepatic cholangiocarcinoma was 44.4%, which was significantly higher than that of adjacent tissues. The increased expression of B7-H4 in intrahepatic cholangiocarcinoma was related to TNM staging, tumor differentiation, lymph node metastasis and poor prognosis (P < 0.05). Survival analysis revealed that the overall survival rate of patients in the B7-H4 high expression group was significantly lower than that in the B7-H4 low expression group (P = 0.005). Cox proportional hazards regression model analysis showed that B7-H4 is an independent prognostic factor for patients with intrahepatic cholangiocarcinoma. Conclusion The expression of B7-H4 is increased in intrahepatic cholangiocarcinoma tissues, and the high expression of B7-H4 is closely related to the malignant pathological characteristics and poor prognosis of ICC patients, which has clinical guiding significance for predicting the prognosis of patients. -
Key words:
- Co-stimulatory molecule B7-H4 /
- Intrahepatic cholangiocarcinoma /
- Pathology /
- Prognosis
-
表 1 肝内胆管癌及癌旁组织中B7-H4的表达[n(%)]
Table 1. Expression of B7-H4 in intrahepatic cholangiocarcinoma and adjacent tissues [n(%)]
组别 高表达 低表达 χ2 P 肝内胆管癌组织 40(44.4) 50(55.6) 15.363 < 0.001 肝内胆管癌癌旁组织 12(13.3) 78(86.7) 表 2 B7-H4在ICC患者的表达与临床病理特征的关系分析[n(%)]
Table 2. Analysis of the relationship between the expression of B7-H4 in ICC patients and clinicopathological characteristics [n(%)]
组别 n 高表达 低表达 χ2 P 性别 男 38 23(60.52) 15(39.48) 1.216 0.146 女 52 17(32.69) 35(67.31) 年龄(岁) ≥ 60 36 20(55.55) 16(44.45) 0.178 0.843 < 60 54 28(51.85) 26(48.15) CEA ≥ 5 62 28(45.16) 34(54.84) 3.249 0.019* < 5 28 12(42.85) 16(57.15) CA19-9(U/mL) ≥ 37 67 24(35.82) 43(64.18) 1.736 0.064 < 37 23 16(69.56) 7(30.44) 肿瘤大小(cm) ≥ 5 66 26(39.39) 40(60.61) 0.162 0.942 < 5 24 14(58.33) 10(41.67) 肿瘤分化 中−高分化 42 14(33.33) 28(66.67) 3.284 0.043* 低分化 48 26(54.16) 22(45.84) HBsAg 阳 60 13(21.66) 47(78.34) 2.196 0.128 阴 30 27(90.00) 3(10.00) 肿瘤数目 多发 65 29(44.61) 36(55.39) 0.419 0.142 单发 25 11(44.00) 14(56.00) TNM 分期 Ⅰ+Ⅱ 56 15(26.78) 41(73.22) 0.246 0.042* Ⅲ+Ⅳ 34 25(73.52) 9(26.48) 淋巴结转移 有 29 13(44.82) 16(55.18) 1.025 0.013* 无 61 27(44.26) 34(55.74) 微血管侵犯 有 32 15(46.87) 17(53.13) 1.118 0.049* 无 58 25(43.10) 33(56.90) 注:*P < 0.05 表 3 90例ICC患者单因素和多因素患者的总生存相关因素分析
Table 3. Analysis of factors related to overall survival of 90 ICC patients with univariate and multivariate patients
组别 OS P HR 95% CI 单因素分析 年龄(≥ 60 岁vs < 60 岁) 1.012 0.846~1.172 0.743 性别(男vs女) 1.142 0.513~2.146 0.677 肿瘤分化(中高分化vs低分化) 1.246 0.552~2.416 0.024 淋巴结转移(有vs无) 2.164 1.251~5.219 0.032 CA19-9(≥ 37 U/mL vs < 37 U/mL) 1.483 1.141~3.843 0.149 CEA(≥ 5 μL/L vs < 5 μL/L) 1.426 0.941~3.426 0.071 B7-H4高表达vs低表达) 3.154 0.946~3.226 0.011 多因素分析 淋巴结转移(有vs 无) 2.162 0.415~8.761 0.249 TNM 分期(Ⅰ+Ⅱvs Ⅲ/Ⅳ) 1.174 0.249~3.428 0.152 CEA(≥ 5 μL/L vs < 5 μL/L) 1.462 0.545~3.247 0.191 微血管侵犯 1.279 1.256~5.012 0.082 肿瘤分化(中高分化vs低分化) 1.637 0.492~2.246 0.324 B7-H4高表达vs低表达) 2.546 1.084~5.256 0.032 -
[1] 胡明,魏雷,谢楠,等. B7-H4与肿瘤发生、发展关系的研究进展[J]. 医学综述,2017,23(14):2760-2764. doi: 10.3969/j.issn.1006-2084.2017.14.012 [2] Xie N,Cai J B,Zhang L,et al. Upregulation of B7-H4 promotes tumor progression of intrahepatic cholangiocarcinoma.[J]. Cell Death & Disease,2017,8(12):3205. [3] Cai J B,Shi G M,Dong Z R,et al. Ubiquitin-specific protease 7 accelerates p14(ARF)degradation by deubiquitinating thyroid hormone receptor-interacting protein 12 and promotes hepatocellular carcinoma progression[J]. Hepatology,2015,61(5):1603-1614. doi: 10.1002/hep.27682 [4] Sica G L,Choi I H,Zhu G,et al. B7-H4,a molecule of the B7 family,negatively regulates T cell immunity[J]. Immunity,2003,18(6):849-861. doi: 10.1016/S1074-7613(03)00152-3 [5] 刘光艺,黄镇,王子卫. 第8版国际抗癌联盟和美国癌症联合委员会胃癌TNM分期系统简介及解读[J]. 腹部外科,2017,30(4):241-245. doi: 10.3969/j.issn.1003-5591.2017.04.002 [6] Qian Y,Hong B,Shen L,et al. B7-H4 enhances oncogenicity and inhibits apoptosis in pancreatic cancer cells.[J]. Cell & Tissue Research,2013,353(1):139-151. [7] Chen Y,Guo G,Guo S,et al. Intracellular B7-H4 suppresses bile duct epithelial cell apoptosis in human primary biliary cirrhosis[J]. Inflammation,2011,34(6):688-697. doi: 10.1007/s10753-010-9280-6 [8] Zhang L,Wu H,Lu D,et al. The costimulatory molecule B7-H4 promote tumor progression and cell proliferation through translocating into nucleus[J]. Oncogene,2013,32(46):5347. doi: 10.1038/onc.2012.600 [9] Jeon Y K,Park S G,Choi I W,et al. Cancer cell-associated cytoplasmic B7-H4 is induced by hypoxia through hypoxia-inducible factor-1α and promotes cancer cell proliferation[J]. Biochem Biophys Res Commun,2015,459(2):277-283. doi: 10.1016/j.bbrc.2015.02.098 [10] Cui L,Gao B O,Cao Z,et al. Downregulation of B7-H4 in the MHCC97-H hepatocellular carcinoma cell line by arsenic trioxide[J]. Molecular Medicine Reports,2016,13(3):2032-2038. doi: 10.3892/mmr.2016.4757 [11] Peng H,Wu W,Yang D,et al. Role of B7-H4 siRNA in proliferation,migration,and invasion of LOVO colorectal carcinoma cell line[J]. Biomed Research International,2015,2015(12):326981. [12] Chen X,Wang L,Wang W,et al. B7-H4 facilitates proliferation of esophageal squamous cell carcinoma cells through promoting IL-6/STAT3 pathway activation[J]. Cancer Science,2016,107(7):944-954. doi: 10.1111/cas.12949 [13] 宋慧,谢炜,练启慧,等. PI3K/AKT信号通路的抑制促进B7-H4分子的核转移[J]. 细胞与分子免疫学杂志,2014,30(11):1121-1124. [14] Kang Q,Zou H,Yang X,et al. Characterization and prognostic significance of mortalin,Bcl-2 and Bax in intrahepatic cholangiocarcinoma[J]. Oncol Lett,2018,15(2):2161-2168. [15] Ke A W,Shi G M,Zhou J,et al. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma[J]. Hepatology ,2009,49(2):491-503. doi: 10.1002/hep.22639 [16] Ke A W,Zhang P F,Shen Y H,et al. Generation and characterization of a tetraspanin CD151/integrin alpha6beta1-binding domain competitively binding monoclonal antibody for inhibition of tumor progression in HCC[J]. Oncotarget ,2016,7(5):6314-6322. doi: 10.18632/oncotarget.6833 [17] Zhang C,Liu L X,Dong Z R,et al. Up-regulation of 14-3-3zeta expression in intrahepatic cholangiocarcinoma and its clinical implications[J]. Tumour Biol,2015,36(3):1781-1789. doi: 10.1007/s13277-014-2780-5 [18] Yao Y,Ye H,Qi Z,et al. B7-H4(B7x)-mediated cross-talk between glioma-initiating cells and macrophages via the IL6/JAK/STAT3 pathway lead to poor prognosis in glioma patients[J]. Clin Cancer Res,2016,22(11):2778-2790. doi: 10.1158/1078-0432.CCR-15-0858 [19] Liu L Z,He Y Z,Dong P P,et al. Protein tyrosine phosphatase PTP4A1 promotes proliferation and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma via the PI3K/AKT pathway[J]. Oncotrget ,2016,7(46):75210-75220. doi: 10.18632/oncotarget.12116 [20] Smith J J,Deane N G,Wu F,et al. Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer[J]. Gastroenterology ,2010,138(3):958-968. doi: 10.1053/j.gastro.2009.11.005 [21] Lan Z,Fu D,Xi M. Serum B7 homologous body 4 for the diagnosis of ovarian cancer in Chinese Han women:A meta-analysis[J]. J Cancer Res Ther ,2018,14(9):433-436. doi: 10.4103/0973-1482.177216 -






 下载:
下载: