Retrospective Analysis of 376 Cases of Adverse Reactions to Antituberculosis Drug
-
摘要:
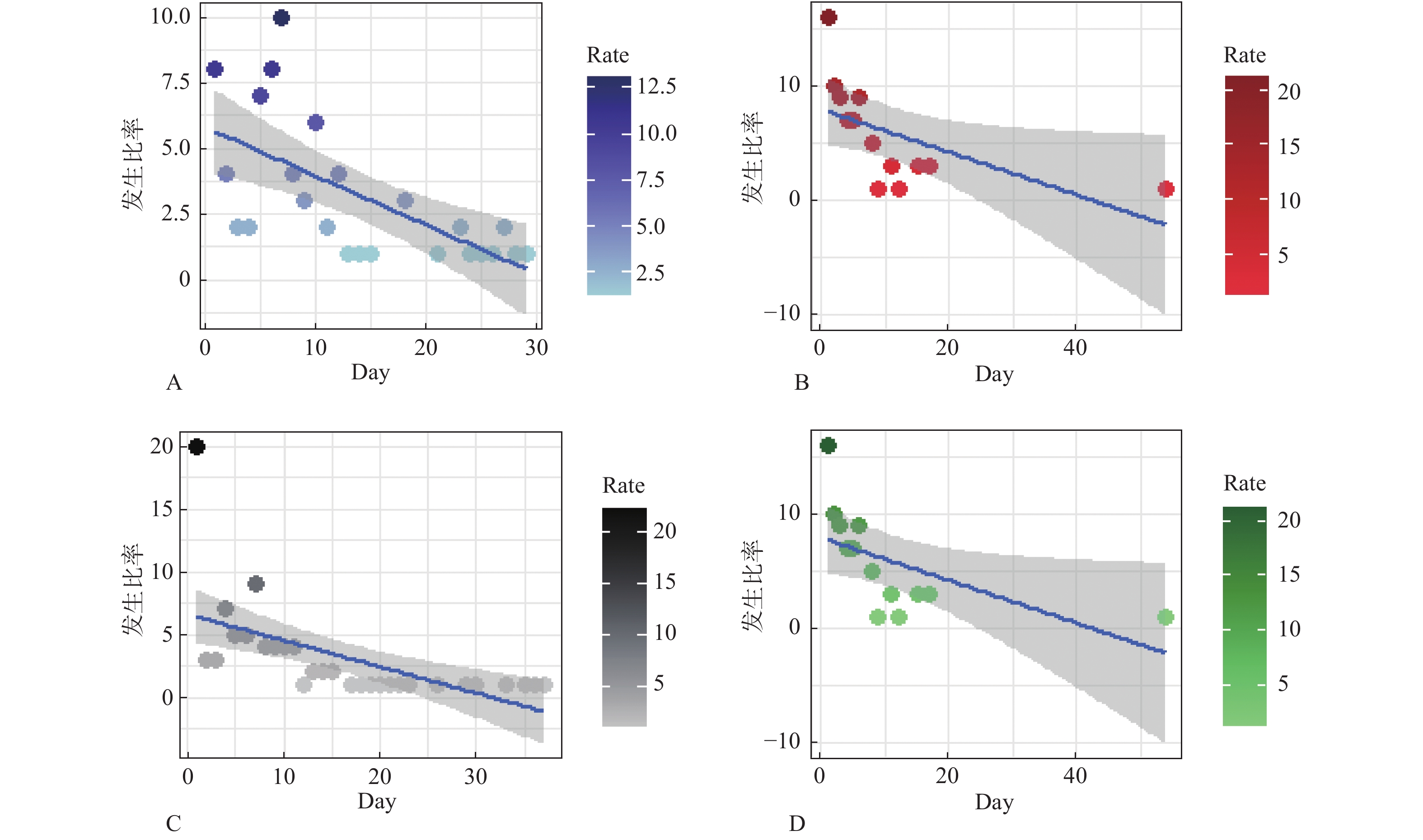
目的 探讨结核患者经抗痨治疗后出现药物不良反应(adverse reaction,ADR)的具体类型、出现时间、年龄构成,为临床合理、安全、有效用药提供一定的参考。 方法 收集昆明市第三人民医院于2015年1月至2019年12月收治的376例因服用抗结核药物而出现不良反应的患者的病历资料,并采用SPSS软件进行数据分析。 结果 患者经抗痨治疗后出现ADR的时间多集中在前10 d,且男性出现ADR的几率高于女性,但差异无统计学意义(P > 0.05)。结核患者在服用利福平(rifampicin,RFP)与异烟肼(isoniazid,INH)后出现肝毒性的几率较高(P < 0.05),结核患者服用吡嗪酰胺(pyrazinamide,PZA)后出现高尿酸血症的几率较高(P < 0.05)。患者服用乙胺丁醇(ethambutol,EMB)后出现皮肤瘙痒的发生率较高(P < 0.05)。 结论 抗结核药物可能导致的ADR多为高尿酸血症、瘙痒、皮疹、肝损伤等。患者初次用药后10d内可能因药物的刺激产生相应的ADR。因此,临床医生在其工作中需重点关注高风险人群以降低患者用药后出现ADR的风险。 Abstract:Objective To explore the specific types, occurrence time and age structure of adverse reaction (ADR) in tuberculosis patients after anti-tuberculosis treatment, so as to provide certain reference for rational, safe and effective drug use in clinical practice. Methods The data of 376 patients with adverse reactions due to antituberculosis drugs admitted to Kunming Third People’ s Hospital from January 2015 to December 2019 were collected, and the data were analyzed using SPSS software. Results The occurrence of ADR in patients after anti-tuberculosis treatment was mostly likely to occur in the first 10 days, and the incidence of ADR in males was higher than that in females, but the difference was not statistically significant. The incidence of hepatotoxicity in TB patients after taking rifampicin (RFP) and isoniazid (INH) was higher (P < 0.05), and the incidence of hyperuricemia in TB patients after taking pyrazinamide (PZA) was higher (P < 0.05). The incidence of pruritus was higher in patients taking ethambutol (EMB) (P < 0.05). Conclusion ADRs caused by antituberculosis drugs are mostly hyperuricemia, pruritus, rash, liver damage, etc. Patients may develop ADRs within 10 days of initial dose. Therefore, clinicians should pay special attention to high-risk groups to reduce the occurence of ADR after medication. -
表 1 肺结核患者总人数(n)
Table 1. The total number of tuberculosis patients (n)
年龄(岁) 男 女 总计 比例(%) 5~14 305 217 522 1.00 15~14 10652 8550 19202 52.00 45~60 6053 3229 9282 25.00 > 60 5187 2962 8149 22.00 合计 22197 14958 37155 构成比(%) 59.00 41.00 表 2 出现ADR肺结核患者人数(n)
Table 2. The number of tuberculosis patients with ADR (n)
年龄(岁) 男 女 合计 构成比(%) 5~14 3 4 7 2.00 15~44 105 95 200 54.00 45~60 72 37 109 29.00 > 60 44 16 60 16.00 合计 224 152 376 构成比(%) 60.00 40.00 表 3 年龄、性别对ADR发生的影响(n)
Table 3. The Influence of age and sex on the occurrence of ADR (n)
内容 发生ADR 未发生ADR χ2 P 性别 男 224 21973 0.004 0.959* 女 152 14806 年龄(岁) 5~14 7 522 9.065 0.028* 15~44 200 19202 45~60 108 9282 > 60 60 8149 *P < 0.05。 表 4 312例常见ADR的临床表现(n)
Table 4. The common clinical manifestations of 312 ADR cases (n)
不良反应 吡嗪酰胺 利福平 异烟肼 乙胺丁醇 高尿酸血症 109 0 0 1 肝损伤 11 28 9 0 胃肠道不适 5 8 3 3 发热 4 13 7 2 皮肤瘙痒 11 38 18 17 关节疼痛 5 0 0 0 白细胞减少 0 17 3 0 总计 145 104 40 23 表 5 312例常见ADR中药物与ADR类型的关系(n)
Table 5. Association between drugs and ADR types in 312 cases (n)
不良反应 药物 发生ADR 未发生ADR χ2 P 高尿酸血症 吡嗪酰胺 109 36 189 < 0.0001* 利福平 0 104 异烟肼 0 40 乙胺丁醇 1 22 肝损伤 吡嗪酰胺 11 134 23 < 0.0001* 利福平 28 76 异烟肼 9 31 乙胺丁醇 0 23 皮肤瘙痒 吡嗪酰胺 11 134 65 < 0.0001* 利福平 38 66 异烟肼 18 22 乙胺丁醇 17 6 发热 吡嗪酰胺 4 141 12 0.05 利福平 13 91 异烟肼 7 33 乙胺丁醇 2 21 *P < 0.05。 表 6 ADR出现时间分布
Table 6. The time distribution of ADR occurrence time
年龄(岁) ADR出现时间(d) 合计(n) 占比(%) 1~7 8~15 16~30 31~90 > 90 ≤18 9 7 1 0 3 20 5.30 18~40 94 31 15 15 8 163 43.40 41~65 85 33 17 10 9 154 40.90 > 66 28 7 2 1 1 39 10.40 合计 216 78 35 26 21 376 100 占比(%) 57.00 21.00 9.00 7.00 6.00 100 -
[1] Kerkhoff A D,Havlir D V. Virtual croi 2020:Tuberculosis and coinfections in hiv infection[J]. Topics In Antiviral Medicine,2020,28(2):455-458. [2] Loutet M G,Davidson J A,Brown T,et al. Acquired resistance to antituberculosis drugs in England,wales,and northern ireland,2000—2015[J]. Emerging Infectious Diseases,2018,24(3):524-533. doi: 10.3201/eid2403.171362 [3] Walker T M,Cruz A L G,Peto T E,et al. Tuberculosis is changing[J]. The Lancet Infectious Diseases,2017,17(4):359-361. doi: 10.1016/S1473-3099(17)30123-8 [4] Wang Y,Xiang X,Huang W W,et al. Association of pxr and car polymorphisms and antituberculosis drug-induced hepatotoxicity[J]. Scientific Reports,2019,9(1):1-9. [5] Migliori G B,Global Tuberculosis Network (GTN). Evolution of programmatic definitions used in tuberculosis prevention and care[J]. Clinical Infectious Diseases,2019,68(10):1787-1789. doi: 10.1093/cid/ciy990 [6] Singla R,Sharma S K,Mohan A,et al. Evaluation of risk factors for antituberculosis treatment induced hepatotoxicity[J]. Indian Journal of Medical Research,2010,132(1):81-87. [7] Bansal A,Agrawal A,Sharma L,et al. A comparative study of active and passive adverse drug reaction reporting systems in terms of false reporting rate[J]. Scripta Medica,2020,51(4):223-230. doi: 10.5937/scriptamed51-29065 [8] 彭惠,申恩瑞,马世武. 吡嗪酰胺诱导的高尿酸血症[J]. 传染病信息,2018,31(4):376-380. doi: 10.3969/j.issn.1007-8134.2018.04.019 [9] Xia H,Van Den Hof S,Cobelens F,et al. Value of pyrazinamide for composition of new treatment regimens for multidrug-resistant mycobacterium tuberculosis in china[J]. Bmc Infectious Diseases,2020,20(1):1-10. doi: 10.1186/s12879-019-4717-5 [10] Pham A Q,Doan A,Andersen M. Pyrazinamide-induced hyperuricemia[J]. Pharmacy And Therapeutics,2014,39(10):695-697. [11] Xu B Y,Tang X D,Chen J,et al. Rifampicin induces clathrin-dependent endocytosis and ubiquitin–proteasome degradation of mrp2 via oxidative stress-activated pkc-erk/jnk/p38 and pi3k signaling pathways in hepg2 cells[J]. Acta Pharmacologica Sinica,2020,41(1):56-64. doi: 10.1038/s41401-019-0266-0 [12] Son C G,Son C G. A severe hepatotoxicity by antituberculosis drug,and its recovery in oriental hospital[J]. Journal of Korean Medicine,2016,37(2):119-124. doi: 10.13048/jkm.16028 [13] Zhang Jin Xin,Chen Juan Juan,Peng Jie. Research progress of anti-tuberculosis drug-induced liver injury[J]. Electronic Journal of Emerging Infectious Diseases,2019,4(3):173-176. [14] 马玉炯,张倩,兀威. 抗结核药物不良反应研究进展[J]. 山东医药,2019,59(32):111-113. doi: 10.3969/j.issn.1002-266X.2019.32.031 [15] 王玉鹏,鲍婕. 异烟肼致肝损伤发病机制的研究进展[J]. 药学实践杂志,2019,37(4):289-293. doi: 10.3969/j.issn.1006-0111.2019.04.001 [16] Sharma P,Kumar A,Singh P. A study of gender differentials in the prevalence of tuberculosis based on nfhs-2 and nfhs-3 data[J]. Indian Journal of Community Medicine:Official Publication of Indian Association of Preventive & Social Medicine,2010,35(2):230-237. [17] 韩珂卿,栾飞,刘道恒,等. 结核病专科医院药品不良反应回顾性分析[J]. 临床肺科杂志,2019,24(8):1459-1462. doi: 10.3969/j.issn.1009-6663.2019.08.025 [18] Kuyinu Y A,Odugbemi B A,Salisu Olatunji S O,et al. Characteristics of mycobacterium tuberculosis positive patients screened for drug-resistant tuberculosis at a tertiary health facility in Lagos,Nigeria[J]. Journal of the National Medical Association,2018,110(1):88-91. doi: 10.1016/j.jnma.2017.04.007 [19] Silva D R,Muñoz Torrico M,Duarte R,et al. Risk factors for tuberculosis:diabetes,smoking,alcohol use,and the use of other drugs[J]. Jornal Brasileiro De Pneumologia,2018,44(2):145-152. doi: 10.1590/s1806-37562017000000443 [20] Carroll M,Lee M,Cai Y,et al. Frequency of adverse reactions to first-and second-line anti-tuberculosis chemotherapy in a korean cohort[J]. The International Journal of Tuberculosis and Lung Disease,2012,16(7):961-966. doi: 10.5588/ijtld.11.0574 -






 下载:
下载: