Determination of Volatile Components of Blaps rhynchoptera by Headspace Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry
-
摘要:
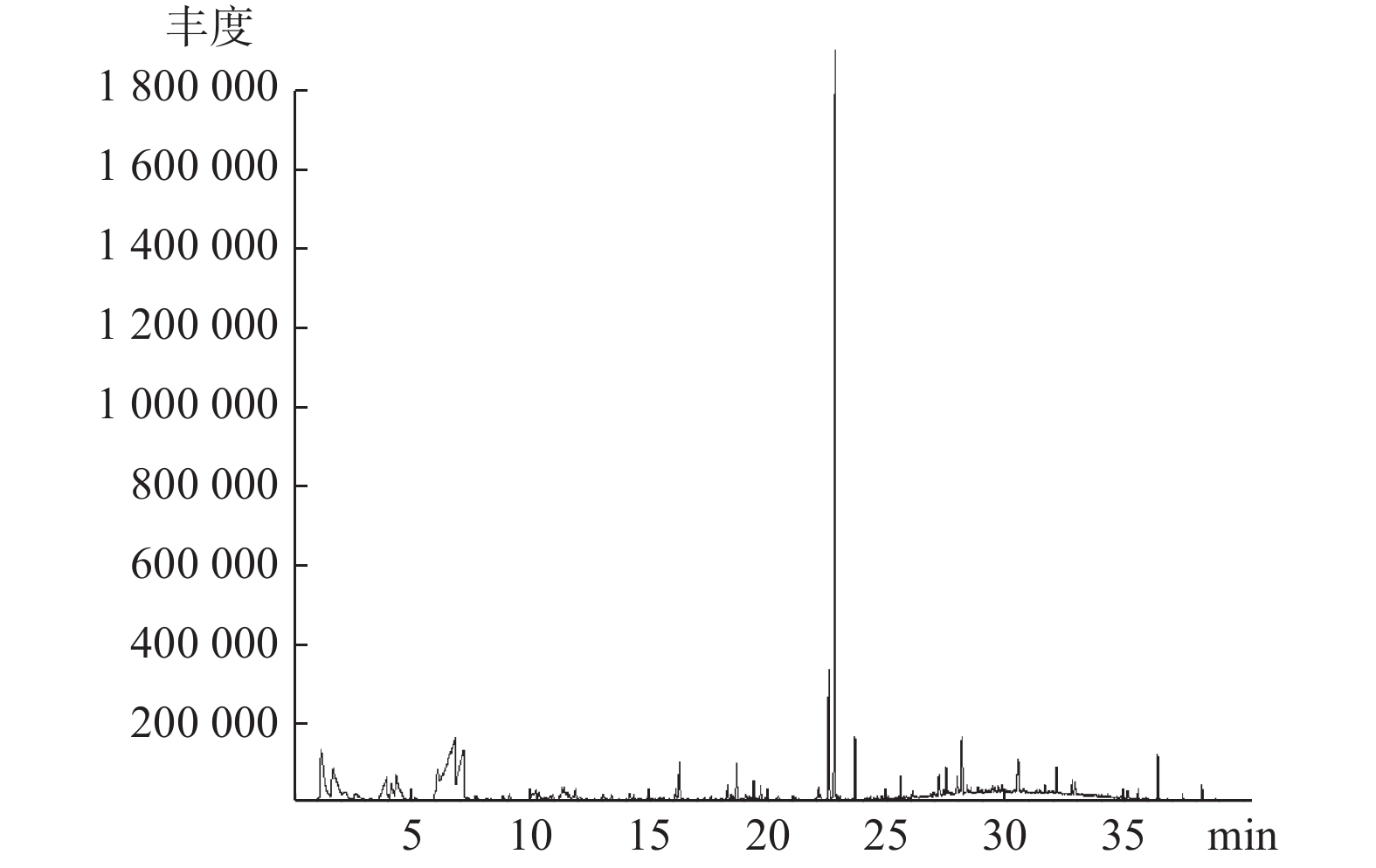
目的 对喙尾琵甲干燥虫体的挥发性化学成分进行分析。 方法 采用顶空固相微萃取方法,结合气质联用技术对其中挥发性成分进行分析和鉴定,通过峰面积归一化法测定各成分的相对含量。 结果 经质谱扫描结合标准谱库检索对比,共鉴定出其中40个成分,占总峰面积的92.80%。其中烃类16种,占总含量的35.73%,以直链和支链烷烃为主;脂肪酸类8种,占总含量的35.87%,以短链脂肪酸为主;此外,还含有胺类、醇类、酯类、醛类、酚类、酮类、醚类等成分。含量在5%以上的组分有十三烷(21.47%)、3-甲基丁酸(16.88%)、2-甲基丁酸(7.67%)、三甲胺(6.59%)和乙酸(5.47%)。 结论 喙尾琵甲干燥虫体中的挥发性成分以烃类和短链脂肪酸类化合物为主,这些成分具有多种生理活性,为药用昆虫资源的进一步开发利用提供参考。 Abstract:Objective To determine the volatile components of the dried Blaps rhynchoptera Fairmaire. Methods Headspace solid phase microextraction (HS-SPME) combined with gas chromatography-mass spectrometry (GC-MS) were used to analysis and identify the volatile components, and the relative content of each compound was calculated using the area normalization method. Results Through mass spectrum scanning combined with mass standard library, 40 components were identified, accounting for 92.80% of the total peak area. Sixteen hydrocarbons were represented by straight-chain and branched alkanes, accounting for 35.73%. Eight fatty acids were represented by short-chain fatty acids, accounted for 35.87%. In addition, it also contained amines, alcohols, esters, aldehydes, phenols, ketones, and ethers. Tridecane, 3-methylbutyric acid, 2-methylbutyric acid, trimethylamine and acetic acid were identified as the major components, accounting for 21.47%, 16.88%, 7.67%, 6.59% and 5.47%, respectively. Conclusion Hydrocarbons and short-chain fatty acids are main volatile components in the dried B. rhynchoptera, which have a variety of pharmacological activities. The results could provide a theoretical basis for the further development and utilization of the medicinal insect resources. -
表 1 喙尾琵甲挥发性成分分析结果
Table 1. Volatile components from Blaps rhynchoptera
序号 保留时间(min) 化合物名称 分子式 分子量 含量(%) 1 1.16 三甲胺 N,N-Dimethylmethylamine C3H9N 59.11 6.59 2 1.67 乙酸 Acetic acid C2H4O2 60.05 5.47 3 2.15 异戊醛 3-Methylbutanal C5H10O 86.13 1.25 4 2.63 丙酸 Propanoic acid C3H6O2 74.08 0.96 5 3.92 异丁酸 Isobutyric acid C4H8O2 88.11 2.92 6 4.11 2,3-丁二醇 2,3-Butanediol C4H10O2 90.12 1.34 7 4.32 1,3-丁二醇 1,3-Butanediol C4H10O2 90.12 2.99 8 6.06 异戊酸乙酯 Ethyl isopentanoate C7H14O2 130.18 2.69 9 6.81 3-甲基丁酸 Isovaleric acid C5H10O2 102.13 16.88 10 7.17 2-甲基丁酸 2-Methyl butyric acid C5H10O2 102.13 7.67 11 9.10 二乙二醇单甲醚 Ethanol,2-(2-methoxyethoxy)- C5H12O3 120.15 0.33 12 10.22 4-甲基戊酸 4-Methylvaleric acid C6H12O2 116.16 1.10 13 10.32 4-甲基戊酸乙酯 Ethyl 4-methylvalerate C8H16O2 144.21 0.51 14 11.30 苯酚 Phenol C6H6O 94.11 0.54 15 11.39 1,2,4-三甲基苯 1,2,4-trimethyl-benzene C9H12 120.19 0.62 16 11.52 己酸 Hexanoic acid C6H12O2 116.16 0.37 17 13.04 N-(2-甲基丙基)乙酰胺 N-(2-Methylpropyl)acetamide C6H13NO 115.17 0.38 18 13.38 泛酰内酯 Pantolactone C6H10O3 130.14 0.29 19 14.15 γ-己内酯 4-Hexalactone C6H10O2 114.14 0.38 20 14.95 2-吡咯烷酮 2-Pyrrolidinone C4H7NO 85.10 0.32 21 16.09 芳樟醇 Linalool C10H18O 154.25 0.47 22 16.25 壬醛 Nonanal C9H18O 142.24 1.58 23 18.66 3,5,5-三甲基环己烯 3,5,5-Trimethy-cyclohexene C9H16 124.22 1.13 24 19.03 萘 Naphthalene C10H8 128.17 0.29 25 19.37 α-松油醇α-Terpineol C10H18O 154.25 0.74 26 19.67 十二烷 Dodecane C12H26 170.33 0.54 27 22.11 壬酸 Nonanoic acid C9H18O2 158.24 0.50 28 22.54 1-十三烯 1-Tridecene C13H26 182.34 3.55 29 22.8 十三烷 Tridecane C13H28 184.36 21.47 30 25.56 十四烷 Tetradecane C14H30 198.39 0.59 31 27.18 2,6,10-三甲基十二烷 2,6,10-Trimethyldodecane C15H32 212.41 0.78 32 27.49 2,6-二(叔丁基)-4-羟基-4-甲基-2,5-环己二烯-1-酮
2,6-Di(tert-butyl)-4-hydroxy-4-methyl-2,5-cyclohexadien-1-oneC15H24O2 236.35 0.80 33 27.94 1-十五烯 1-Pentadecene C15H30 210.40 0.83 34 28.14 十五烷 Pentadecane C15H32 212.41 2.60 35 28.37 α-法尼烯 α-Farnesene C15H24 204.35 0.45 36 30.52 十六烷 Hexadecane C16H34 226.44 1.37 37 31.65 2,6,10-三甲基十五烷2,6,10-Trimethylpentadecane C18H38 254.49 0.35 38 32.78 十七烷 Heptadecane C17H36 240.47 0.41 39 32.91 姥鲛烷 pentadecane,2,6,10,14-tetramethyl- C19H40 268.52 0.40 40 35.12 植烷 Hexadecane,2,6,10,14-tetramethyl- C20H42 282.55 0.35 -
[1] 任国栋, 张润志, 石福明. 昆虫分类与多样性[M]. 北京: 中国农业科学技术出版社, 2005: 176. [2] 赵敏,冯颖,陈晓鸣,等. 喙尾琵甲(鞘翅目:拟步甲科)形态记述及生物学特性观察[J]. 环境昆虫学报,2009,31(4):348-355. [3] Zhang X,Feng Y,Ding W F,et al. A new continuous cell line from Blaps rhynchoptera Fairmaire (Coleoptera:Tenebrionidae)[J]. In Vitro Cellular & Developmental Biology-Animal,2015,51(2):151-156. [4] 张兰胜,夏从龙,杨永寿. 彝药喙尾琵琶甲的研究进展[J]. 时珍国医国药,2009,20(12):3113-3114. doi: 10.3969/j.issn.1008-0805.2009.12.104 [5] Xiao H,Dong J W,Zhou D J,et al. Cytotoxicity of the defensive secretion from the medicinal insect Blaps rynchopetera [J]. Molecules,2017,23(1):10. doi: 10.3390/molecules23010010 [6] 郭明磊,刘建宏. 喙尾琵琶甲化学防御物质气相色谱-质谱分析及抑菌试验[J]. 湖北农业科学,2016,55(5):1271-1273. [7] 罗建蓉,何江波,张桢,等. 药用昆虫喙尾琵琶甲化学成分研究[J]. 中成药,2010,32(11):2013-2014. doi: 10.3969/j.issn.1001-1528.2010.11.051 [8] 罗情,蔡乐,巫秀美,等. 药用昆虫喙尾琵琶甲中环肽类成分研究[J]. 中草药,2015,46(16):2381-2384. [9] 贾力扬,李启艳. 喙尾琵琶甲化学成分及药理应用的研究进展[J]. 中医药学报,2018,46(6):118-122. [10] 罗建蓉,张 桢,杨永寿,等. 药用昆虫喙尾琵琶甲油脂化合物的气相色谱-质谱分析[J]. 时珍国医国药,2010,21(1):12-13. doi: 10.3969/j.issn.1008-0805.2010.01.007 [11] 肖怀. 云南彝族药用昆虫喙尾琵琶甲物质基础及抗肿瘤相关活性研究[D]. 昆明: 云南大学博士学位论文, 2018. [12] 罗建蓉,车逸豪,苏锦松,等. 彝药琵琶甲石油醚部位化学成分研究[J]. 中药材,2020,43(12):2924-2927. [13] Bergmann J,González A,Zarbin P H G. Insect pheromone research in South America[J]. Journal of the Brazilian Chemical Society,2009,20(7):1206-1219. doi: 10.1590/S0103-50532009000700003 [14] 李凯,陈列忠,陈建明,等. 褐飞虱遇险释放挥发性物质的提取及成分分析[J]. 应用昆虫学报,2013,50(5):1354-1363. doi: 10.7679/j.issn.2095-1353.2013.186 [15] 王可鑫,姜宁,张爱忠. 短链脂肪酸介导的宿主肠道免疫调控机制[J]. 动物营养学报,2020,32(4):1544-1550. doi: 10.3969/j.issn.1006-267x.2020.04.010 [16] Nogal A,Valdes A M,Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health[J]. Gut Microbes,2021,13(1):1-24. [17] Canfora E,Jocken J,Blaak E. Short-chain fatty acids in control of body weight and insulin sensitivity[J]. Nature Reviews Endocrinology,2015,11(10):577-591. doi: 10.1038/nrendo.2015.128 [18] Wouw M V D,Boehme M,Lyte J M. Short-chain fatty acids:microbial metabolites that alleviate stress-induced brain–gut axis alterations[J]. The Journal of Physiology,2018,596(20):4923-4944. doi: 10.1113/JP276431 -






 下载:
下载: