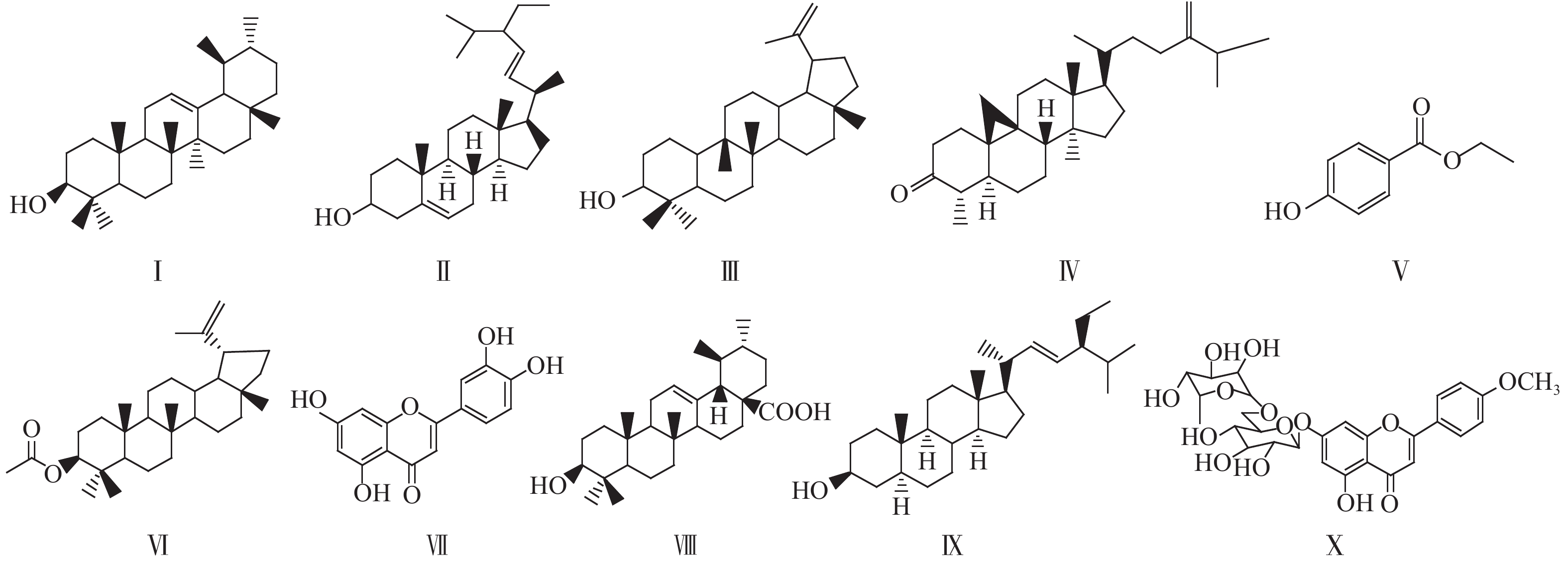
|
[1]
|
郭雷,朱文成,刘超. 密蒙花化学成分及生物活性研究进展[J]. 食品研究与开发,2012,33(7):222-225. doi: 10.3969/j.issn.1005-6521.2012.07.063
|
|
[2]
|
杨东方,胡云飞,丁倩倩,等. 密蒙花的化学成分及质量控制方法研究进展[J]. 中国现代应用药学,2019,36(22):2870-2875.
|
|
[3]
|
Yan Huang,Xu Wu,Jianwen Wen,et al. A Study on the Chemical Constituents of Pileasinofasciata C. J. Chen[J]. Medicinal Plant,2017,8(3):1-4.
|
|
[4]
|
薛鹏辉,段静诗,丁丽琴,等. 鹅不食草中甾体及酚类化学成分研究[J]. 中国药物化学杂志,2020,30(6):340-346.
|
|
[5]
|
孙志青,刘立云,孙岩,等. 血芙蓉化学成分的研究[J]. 中成药,2020,42(4):932-938. doi: 10.3969/j.issn.1001-1528.2020.04.021
|
|
[6]
|
吴贵辉,陈艳,郑黎花,等. 黔产杜仲化学成分研究[J]. 中药材,2015,38(5):980-984.
|
|
[7]
|
张鹏耀,舒任庚,严喜鸾,等. 山蜡梅化学成分研究[J]. 中国药学杂志,2017,52(18):1594-1596.
|
|
[8]
|
Chen Hui,Li Yu Jie,Sun Yan Jun,et al. Antihyperlipidemic glycosides from the root bark of Lyciumchinense.[J]. Natural Product Research,2019,33(18):2655-2661. doi: 10.1080/14786419.2018.1466125
|
|
[9]
|
何丽丽,姚彩云,闫炳雄,等. 壮药山风中的黄酮类化学成分研究[J]. 中国现代中药,2019,21(8):1016-1020.
|
|
[10]
|
孙彦君,陈豪杰,高美玲,等. 小驳骨中萜类化学成分研究[J]. 中草药,2020,51(2):293-298.
|
|
[11]
|
刘宝,胡飞龙,雷福厚,等. 灰色紫金牛化学成分研究[J]. 中药材,2019,42(3):560-562.
|
|
[12]
|
郑畅,阮静雅,瞿璐,等. 密蒙花中黄酮类成分的分离与鉴定[J]. 中国药物化学杂志,2018,28(1):52-57.
|






 下载:
下载: