The Application Value of Dry Fluorescent Luminescence in The Diagnosis and Treatment of HBV Infection
-
摘要:
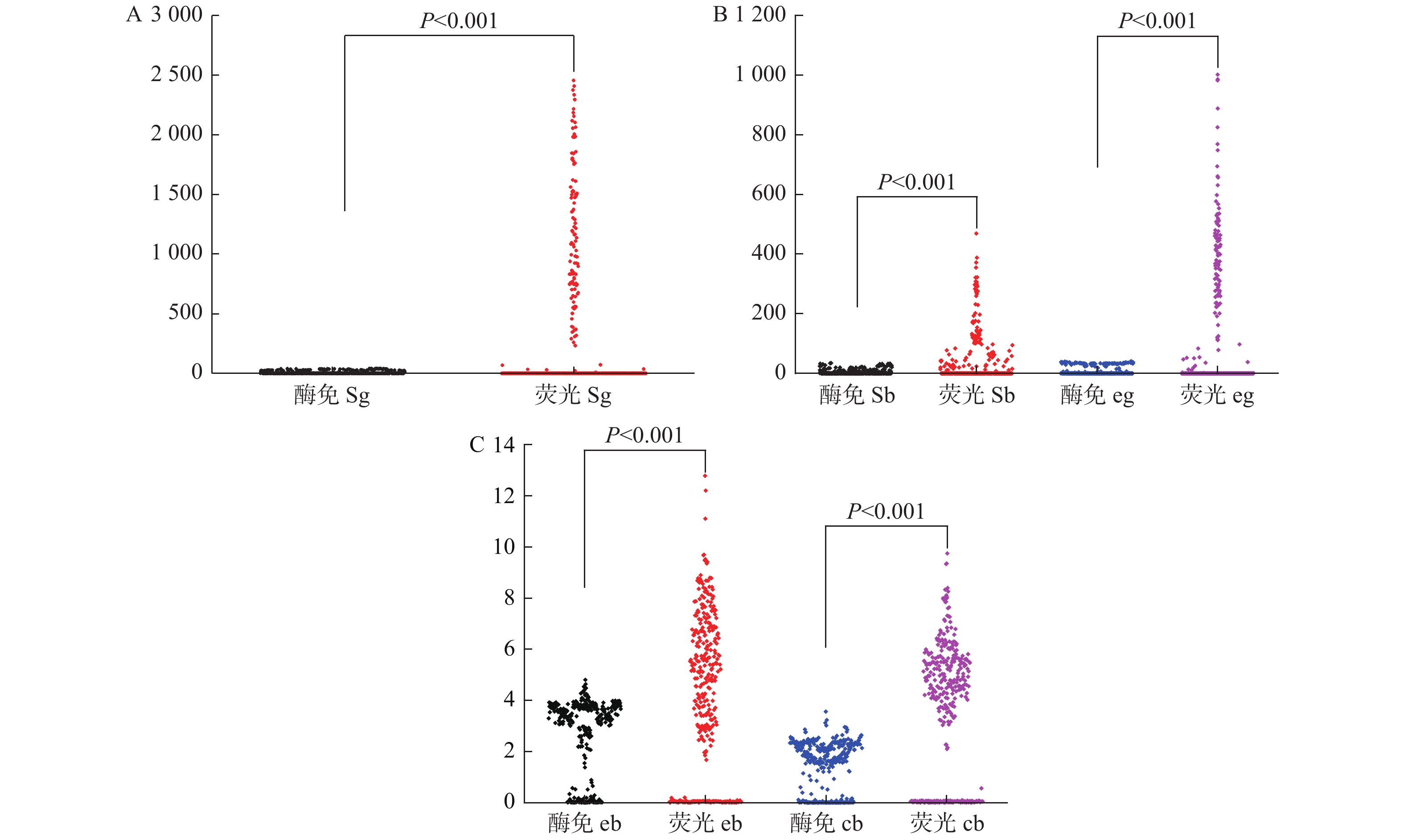
目的 分析评价干式荧光发光法在HBV感染诊疗中的应用价值。 方法 收集2020年6月1日至2020年12月31日昆明市第三人民医院肝病科就诊患者血清样本397例,其中360例样本采用干式荧光发光法、酶联免疫法进行HBsAg、HBsAb、HBeAg、HBeAb和HBcAb五项检测,经电化学发光法检测为HBsAg阳性HBeAg血清学转换期的37例样本采用干式荧光发光法、酶联免疫法和胶体金法3种检测技术进行HBsAg、HBeAg和HBeAb 3项检测,对比分析干式荧光发光法在HBV感染诊疗中各检测项目的阳性符合率、阴性符合率、总符合率等指标。 结果 酶联免疫法S/C值与干式荧光发光法S/C值趋势基本一致,呈正相关(γ = 0.819,0.913,0.906,0.732,0.842,P < 0.001)。2种检测技术HBV5项的阳性符合率、阴性符合率、总符合率均 > 90%,具有较高的一致性(Kappa = 0.949,0.944,0.955,0.842,0.949)。37例HBsAg阳性HBeAg血清学转换期的血清样本检测结果显示干式荧光发光法与罗氏电化学发光HBeAb阳性符合率100%(37/37)远远高于酶联免疫法18.92%(7/37)和胶体金法5.40%(2/37),差异统计学意义( $ {\chi ^2}$ = 13.714,22.752,P < 0.001)。结论 干式荧光发光法能有效的反映出HBeAg血清学转换期,常规HBV筛查试验与酶联免疫法具有较高的一致性,同时具备检测时长短、无频繁手工操作、操作简便等优点,有很好的应用前景。 Abstract:Objective To analyze and evaluate the application value of dry fluorescent luminescence in the diagnosis and treatment of HBV infection. Methods A total of 397 serum samples were collected from patients attending Liver Diseases Department of the Third People’ s Hospital of Kunming from June 1, 2020 to December 31, 2020. Among these samples, 360 were detected for HBsAg, HBsAb, HBeAg, HBeAb and HBcAb by dry fluorescent luminescence method and ELISA, and 37 HBsAg positive samples in HBeAg seroconversion period detected by electrochemiluminescence method were tested for HBsAg, HBeAg and HBeAb by three methods: dry fluorescent luminescence method, enzyme-linked immunosorbent assay and colloidal gold method. The positive coincidence rate, negative coincidence rate and total coincidence rate of each test item by dry fluorescent luminescence method in diagnosis and treatment of HBV infection were compared and analyzed. Results The S/C value of ELISA was consistent with the trend of S/C value of dry fluorescent luminescence method, showing a certain positive correlation (r = 0.819, 0.913, 0.906, 0.732, 0.842, P < 0.001). The positive coincidence rate, negative coincidence rate and total coincidence rate of the five items of HBV by the two detection techniques were more than 90%, with high consistency (Kappa = 0.949, 0.944, 0.955, 0.842, 0.949). The results of 37 serum samples from HBsAg positive HBeAg seroconversion period showed that the coincidence rate between dry fluorescent luminescence method and Roche electrochemiluminescence HBeAb was 100% (37/37), much higher than that of ELISA 18.92% (7/37) and colloidal gold 5.40% (2/37). Conclusion Dry fluorescent luminescence method can effectively reflect the serological conversion period of HBeAg. Conventional HBV screening test and enzyme-linked immunosorbent assay have a high consistency. At the same time, it has the advantages of short detection time, no frequent manual operation and simple operation, so it has a good application prospect. -
Key words:
- Dry fluorescent luminescence /
- Hepatitis B virus /
- Accuracy rate
-
表 1 酶联免疫法与干式荧光发光法S/C值比较分析(n = 360)(
$\bar x \pm s$ )Table 1. Comparison of S/C value of ELISA and dry fluorescent luminescence (n = 360)(
$\bar x \pm s$ )检测技术 HBsAg HBsAb HBeAg HBeAb HBcAb 酶联免疫法 9.23 ± 13.69 4.37 ± 8.00 9.15 ± 14.66 2.58 ± 1.58 1.45 ± 0.99 干式荧光发光法 362.21 ± 636.71 39.64 ± 81.58 110.57 ± 205.56 3.99 ± 3.12 3.52 ± 2.67 t 10.516 8.161 9.338 7.698 13.771 p < 0.001* *P < 0.05。 表 2 2种检测技术比较分析(n = 360)
Table 2. Comparison of two types of detection technology (n = 360)
2种检测技术 阳性符合率(%)(95%CI) 阴性符合率(%)(95%CI) 总符合率(%)(95%CI) Kappa系数 HBsAg 96.55(90.88~98.89) 98.36(95.58~99.47) 97.78(92.89~99.09) 0.949 HBsAb 93.55(87.28~96.97) 99.58(97.29~99.98) 97.79(92.93~99.11) 0.944 HBeAg 94.74(88.43~97.84) 99.59(97.40~99.98) 98.06(94.83~99.29) 0.955 HBeAb 98.97(93.57~99.94) 91.25(87.00~94.25) 93.33(87.03~95.79) 0.842 HBcAb 99.12(94.45~99.95) 97.17(94.00~98.75) 97.78(92.94~99.19) 0.949 表 3 3种检测技术符合率比较分析(n = 37)
Table 3. Comparison of three types of detection technology of accuracy rate (n = 37)
检测技术 阳性符合率(%) HBsAg HBeAg HBeAb 酶联免疫法 100 59.46 18.92 干式荧光发光法 100 62.16 100 胶体金法 97.30 0 5.40 P < 0.001* *P < 0.05。 -
[1] Li M,Zu J. The review of differential equation models of HBV infection dynamics[J]. J Virol Methods,2019,266(6):103-113. [2] Nasir M,Wu G Y. HEV and HBV Dual Infection:A Review[J]. J Clin Transl Hepatol,2020,8(3):313-321. [3] Jia Y,Shu X,Yang X,et al. Enhanced therapeutic effects of umbilical cord mesenchymal stem cells after prolonged treatment for HBV-related liver failure and liver cirrhosis[J]. Stem Cell Res Ther,2020,11(1):277. doi: 10.1186/s13287-020-01787-4 [4] Kanda T,Goto T,Hirotsu Y,et al. Molecular Mechanisms Driving Progression of Liver Cirrhosis towards Hepatocellular Carcinoma in Chronic Hepatitis B and C Infections:A Review[J]. Int J Mol Sci,2019,20(6):1358. doi: 10.3390/ijms20061358 [5] 陈忠伟,樊怡昕,胡汉宁,等. 慢性乙型肝炎相关原发性肝癌的生物标志物:新的发现和未来之路[J]. 湖北大 学学报(自然科学版),2021,43(1):33-40,47. [6] Chen Y,Tian Z. HBV-Induced Immune Imbalance in the Development of HCC[J]. Front Immunol,2019,27(10):2048. [7] 中华医学会肝病学分会,中华医学会感染病学分会. 慢性乙型肝炎防治指南(2015更新版)[J]. 中华肝脏病杂志,2015,23(12):888-905. doi: 10.3760/cma.j.issn.1007-3418.2015.12.002 [8] Liu Y,Wu C,Chen R,et al. Molecular cloning and phenotypic analysis of drug-resistance mutants with relevant S-region variants of HBV for a patient during 189-month anti-HBV treatment[J]. Antivir Ther,2019,24(4):237-246. doi: 10.3851/IMP3305 [9] 谢青,宁琴,王贵强,等. 乙型肝炎临床治愈策略:抗病毒药物与免疫调节治疗[J]. 中华肝脏病杂志,2020,28(8):644-648. doi: 10.3760/cma.j.cn501113-20200722-00410 [10] Li H Y,Jia W N,Li X Y,et al. Advances in detection of infectious agents by aptamer-based technologies[J]. Emerg Microbes Infect,2020,9(1):1671-1681. doi: 10.1080/22221751.2020.1792352 [11] Furuta M,Tanaka H,Shiraishi Y,et al. Characterization of HBV integration patterns and timing in liver cancer and HBV-infected livers[J]. Oncotarget,2018,9(38):25075-25088. doi: 10.18632/oncotarget.25308 [12] 云南省2013-2017年农村地区育龄人群HBsAg筛查结果分析[J]. 中华流行病学杂志, 2020, 41(9): 1522-1526. [13] 王新梅,臧亮,邓雪莲,等. 化学发光和酶免2种检测系统检测HBsAg情况分析[J]. 中国输血杂志,2020,33(3):222-226. [14] 钟曼华,陈燕鸿,王小凤,等. HBeAg阳性CHB患者核苷类似物治疗停药后复发情况及其影响因素分析[J]. 山东医药,2021,61(10):60-62. doi: 10.3969/j.issn.1002-266X.2021.10.014 [15] Ouyang Y,Fu X,Peng S,et al. Plasma miR-146a predicts serological conversion of hepatitis B e-antigen(HBeAg)in chronic hepatitis B patients treated with nucleotide analogs[J]. Ann Transl Med,2019,7(18):449. doi: 10.21037/atm.2019.08.72 -







 下载:
下载: