Mechanism of miR-373 on Behaviors of Depression-mice via P2X7R
-
摘要:
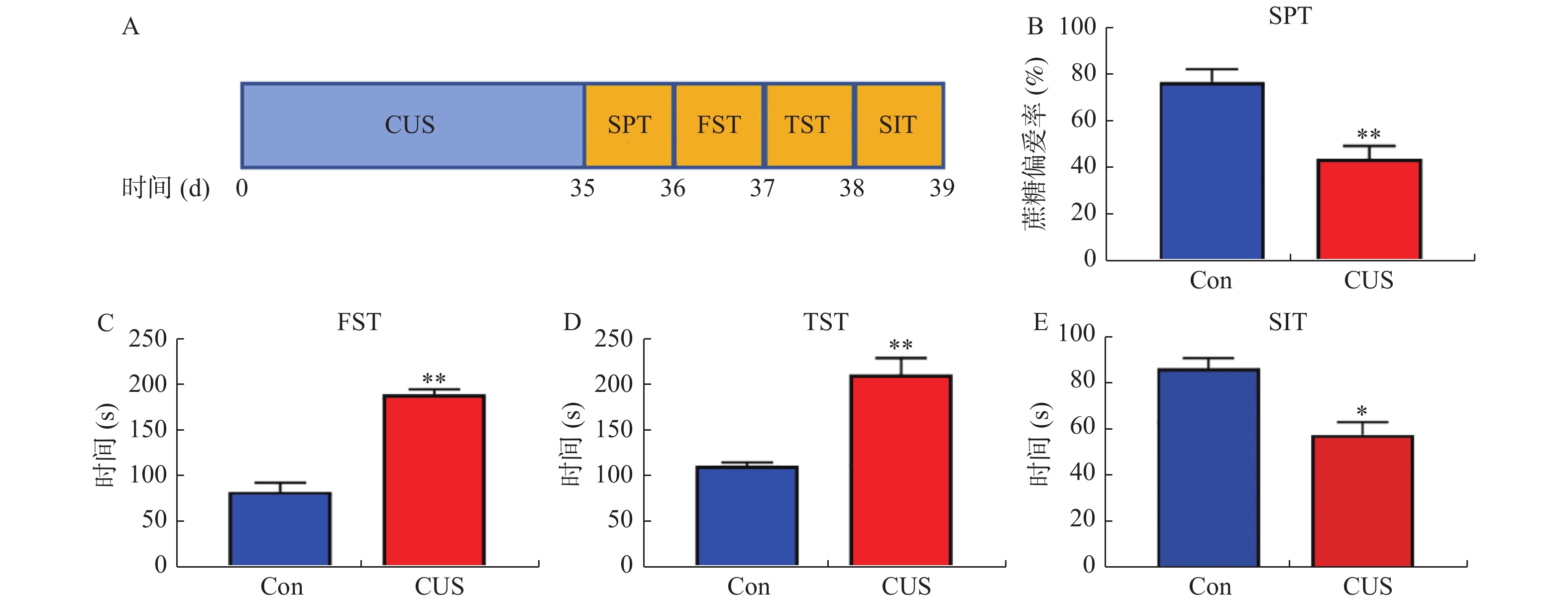
目的 探讨miR-373对抑郁症小鼠模型抑郁样行为,小胶质细胞激活和焦亡的影响。 方法 采用慢性不可预知应激构建抑郁症小鼠模型。蔗糖偏好,强迫游泳,尾部悬挂和社会实验评估小鼠的抑郁样行为。免疫荧光双染小胶质细胞标志物Iba-1和小胶质细胞活化标志物OX-42以评估小鼠海马层中小胶质细胞增殖和活化状态。TUNEL试剂盒检测小胶质细胞的凋亡。荧光定量PCR检测miR-373的表达水平。蛋白质印迹检测P2X7R和细胞焦亡相关蛋白的表达水平。双萤光素酶报告基因实验验证miR-373和P2X7R的靶向关系。 结果 慢性不可预知应激处理的小鼠,蔗糖偏好度和社交时间显著下调,并且强迫游泳和尾部悬挂不动时间显著增加(P < 0.05)。miR-373在抑郁症模型小鼠中异常高表达,且能够缓解小鼠的抑郁样行为(P < 0.05)。miR-373靶向负调控小鼠海马层小胶质细胞中P2X7R的表达水平(P < 0.01)。miR-373抑制小鼠海马层中小胶质细胞增殖和激活(P < 0.01)。miR-373抑制小胶质细胞中Caspase-1,C-caspase-1,NLRP3,IL-1β和IL-18表达,并抑制小胶质细胞凋亡(P < 0.05)。 结论 miR-373通过靶向抑制P2X7R的表达,从而缓解抑郁症小鼠模型的抑郁样行为,并抑制其海马层中小胶质细胞活化和焦亡。 Abstract:Objective To explore the effect of miR-373 on depression-like behavior, microglia activation and pyrolysis in depression mice. Methods We Used chronic unpredictable stress to establish depression mice model. Sucrose preference, forced swimming, tail suspension, and Social interaction test were evaluate depression-like behaviors. Immunofluorescence double-stained microglia marker Iba-1 and microglia activation marker OX-42 were used to evaluate the proliferation and activation of microglia in the hippocampus of mice. TUNEL kit was used to detect microglia apoptosis. RT-qPCR was used to detect miR-373 expression. Western blotting was used to detect the expression of P2X7R and pyrolysis-related proteins. The targeting relationship between miR-373 and P2X7R was verified by dual-luciferase reporter gene assay. Results In mice treated with chronic unpredictable stress, sucrose preference and social time were significantly reduced, and immobility time of forced swimming and tail suspension was significantly increased (P < 0.05). miR-373 washighly expressed, and alleviated depression-like behavior in depression model mice (P < 0.05). miR-373 targeted and negatively regulated P2X7R expression in microglia ofmice hippocampus (P < 0.01). miR-373 inhibited the proliferation and activation of microglia (P < 0.01). miR-373 inhibited caspase-1, c-caspase-1, NLRP3, IL-1β and IL-18 expression, and the apoptosis of microglia (P < 0.05). Conclusion ImiR-373 inhibits P2X7R expression to alleviate depression-like behavior, and inhibit the activation and pyroptosis of microglia in the hippocampus of depression mice. -
Key words:
- Depression /
- Depression-like behavior /
- Microglia /
- Pyroptosis /
- miR-373 /
- P2X7R
-
图 2 miR-373缓解抑郁症模型小鼠抑郁样行为
A和B:RT-qPCR检测小鼠中miR-373表达水平的结果。抑郁症小鼠模型尾静脉注射ago-miR及ago-miR-373,测量抑郁样行为的结果:C:SPT;D:FST;E:TST;F:SIT。注:图2A,与Con组比较,*P < 0.05,**P < 0.01;图2B,与ago-miR(无意义序列)比较,*P < 0.05,**P < 0.01;图2C-F,与CUS组比较,*P < 0.05,**P < 0.01。
Figure 2. miR-373 alleviates depression-like behavior in depression model mice
表 1 RT-qPCR引物序列
Table 1. RT-qPCR Primer Series
目标 正向引物(5′-3′) 反向引物(5′-3′) miR-373 ACUCAAAAUGGGGGCGGAAAGC CAGTGCGTGTCGTGGAGT U6 CCCTTCGGGGACATCCGATA TTTGTGCGTGTCATCCTTGC caspase-1 TGGATCTTCACAAGGGCGAC CAACACAGCAACCAGCAGAC NLRP3 CAGGATGGCTCTCGCTTCAT CTGACAGAACCGTGGGACTC IL-1β TGCCACCTTTTGACAGTGATG TTCTTGTGACCCTGAGCGAC IL-18 ACAGGACTGCCATCTTCTGC ATTGTTCCTGGGCCAAGAGG β-actin TTCCAGCCTTCCTTCTTG TGTCAACGTCACACTTCA -
[1] Malhi G S,Mann J J. Depression[J]. Lancet,2018,392(10161):2299-2312. doi: 10.1016/S0140-6736(18)31948-2 [2] Cruz-Pereira J S,Rea K,Nolan Y M,et al. Depression's Unholy Trinity:Dysregulated Stress,Immunity,and the Microbiome[J]. Annu Rev Psychol,2020,71:49-78. doi: 10.1146/annurev-psych-122216-011613 [3] Spellman T,Liston C. Toward Circuit Mechanisms of Pathophysiology in Depression[J]. Am J Psychiatry,2020,177(5):381-390. doi: 10.1176/appi.ajp.2020.20030280 [4] Dubovsky S L,Ghosh B M,Serotte J C,et al. Psychotic Depression:Diagnosis,Differential Diagnosis,and Treatment[J]. Psychother Psychosom,2021,90(3):160-177. doi: 10.1159/000511348 [5] Hammen C. Risk Factors for Depression:An Autobiographical Review[J]. Annu Rev Clin Psychol,2018,14:1-28. doi: 10.1146/annurev-clinpsy-050817-084811 [6] Menke A,Nitschke F,Hellmuth A,et al. Stress impairs response to antidepressants via HPA axis and immune system activation[J]. Brain Behav Immun,2021,93:132-140. doi: 10.1016/j.bbi.2020.12.033 [7] Miller A H,Raison C L. The role of inflammation in depression:from evolutionary imperative to modern treatment target[J]. Nat Rev Immunol,2016,16(1):22-34. doi: 10.1038/nri.2015.5 [8] Beurel E,Toups M,Nemeroff C B. The Bidirectional Relationship of Depression and Inflammation:Double Trouble[J]. Neuron,2020,107(2):234-256. doi: 10.1016/j.neuron.2020.06.002 [9] Nerurkar L,Siebert S,Mcinnes I B,et al. Rheumatoid arthritis and depression:an inflammatory perspective[J]. Lancet Psychiatry,2019,6(2):164-173. doi: 10.1016/S2215-0366(18)30255-4 [10] Yirmiya R,Rimmerman N,Reshef R. Depression as a microglial disease[J]. Trends Neurosci,2015,38(10):637-658. doi: 10.1016/j.tins.2015.08.001 [11] Jia X,Gao Z,Hu H. Microglia in depression:current perspectives[J]. Sci China Life Sci,2020. [12] Winkle M,El-Daly S M,Fabbri M,et al. Noncoding RNA therapeutics-challenges and potential solutions[J]. Nat Rev Drug Discov,2021,20(8):629-651. doi: 10.1038/s41573-021-00219-z [13] Makarova J,Turchinovich A,Shkurnikov M,et al. Extracellular miRNAs and Cell-Cell Communication:Problems and Prospects[J]. Trends Biochem Sci,2021,46(8):640-651. doi: 10.1016/j.tibs.2021.01.007 [14] Allen L,Dwivedi Y. MicroRNA mediators of early life stress vulnerability to depression and suicidal behavior[J]. Mol Psychiatry,2020,25(2):308-320. doi: 10.1038/s41380-019-0597-8 [15] Van Den Berg M M J,Krauskopf J,Ramaekers J G,et al. Circulating microRNAs as potential biomarkers for psychiatric and neurodegenerative disorders[J]. Prog Neurobiol,2020,185:101732. doi: 10.1016/j.pneurobio.2019.101732 [16] Surget A,Wang Y,Leman S,et al. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal[J]. Neuropsychopharmacology,2009,34(6):1363-80. doi: 10.1038/npp.2008.76 [17] Shen J,Li Y,Qu C,et al. The enriched environment ameliorates chronic unpredictable mild stress-induced depressive-like behaviors and cognitive impairment by activating the SIRT1/miR-134 signaling pathway in hippocampus[J]. J Affect Disord,2019,248:81-90. doi: 10.1016/j.jad.2019.01.031 [18] Li B,Han L,Cao B,et al. Use of magnoflorine-phospholipid complex to permeate blood-brain barrier and treat depression in the CUMS animal model[J]. Drug Deliv,2019,26(1):566-574. doi: 10.1080/10717544.2019.1616236 [19] Liu Z,Yang J,Fang Q,et al. MiRNA-199a-5p targets WNT2 to regulate depression through the CREB/BDNF signaling in hippocampal neuron[J]. Brain Behav,2021,11(8):e02107. [20] Yang J,Sun J,Lu Y,et al. Revision to psychopharmacology mRNA and microRNA profiles are associated with stress susceptibility and resilience induced by psychological stress in the prefrontal cortex[J]. Psychopharmacology(Berl),2020,237(10):3067-3093. doi: 10.1007/s00213-020-05593-x [21] Mclarnon J G. Roles of purinergic P2X(7)receptor in glioma and microglia in brain tumors[J]. Cancer Lett,2017,402:93-99. doi: 10.1016/j.canlet.2017.05.004 [22] Illes P,Khan T M,Rubini P. Neuronal P2X7 Receptors Revisited:Do They Really Exist?[J]. J Neurosci,2017,37(30):7049-7062. doi: 10.1523/JNEUROSCI.3103-16.2017 [23] Yue N,Huang H,Zhu X,et al. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors[J]. J Neuroinflammation,2017,14(1):102. doi: 10.1186/s12974-017-0865-y [24] Albert P R,Le François B,Vahid-Ansari F. Genetic,epigenetic and posttranscriptional mechanisms for treatment of major depression:the 5-HT1A receptor gene as a paradigm[J]. J Psychiatry Neurosci,2019,44(3):164-176. doi: 10.1503/jpn.180209 [25] Yoshino Y,Roy B,Dwivedi Y. Differential and unique patterns of synaptic miRNA expression in dorsolateral prefrontal cortex of depressed subjects[J]. Neuropsychopharmacology,2021,46(5):900-910. doi: 10.1038/s41386-020-00861-y [26] Roy B,Dunbar M,Shelton R C,et al. Identification of MicroRNA-124-3p as a Putative Epigenetic Signature of Major Depressive Disorder[J]. Neuropsychopharmacology,2017,42(4):864-875. doi: 10.1038/npp.2016.175 [27] Xu J,Wang R,Liu Y,et al. FKBP5 and specific microRNAs via glucocorticoid receptor in the basolateral amygdala involved in the susceptibility to depressive disorder in early adolescent stressed rats[J]. Journal of Psychiatric Research,2017,95:102-113. doi: 10.1016/j.jpsychires.2017.08.010 [28] Li C,Feng S,Chen L. MSC-AS1 knockdown inhibits cell growth and temozolomide resistance by regulating miR-373-3p/CPEB4 axis in glioma through PI3K/Akt pathway[J]. Mol Cell Biochem,2021,476(2):699-713. doi: 10.1007/s11010-020-03937-x [29] Peng T,Wang T,Liu G,et al. Effects of miR-373 Inhibition on Glioblastoma Growth by Reducing Limk1 In Vitro[J]. J Immunol Res,2020,2020:7671502. [30] Slowik A,Lammerding L,Hoffmann S,et al. Brain inflammasomes in stroke and depressive disorders:Regulation by oestrogen[J]. J Neuroendocrinol,2018,30(2):9-12. [31] Li Y,Song W,Tong Y,et al. Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis[J]. J Neuroinflammation,2021,18(1):1. doi: 10.1186/s12974-020-02040-8 [32] Tian D D,Wang M,Liu A,et al. Antidepressant Effect of Paeoniflorin Is Through Inhibiting Pyroptosis CASP-11/GSDMD Pathway[J]. Mol Neurobiol,2021,58(2):761-776. doi: 10.1007/s12035-020-02144-5 [33] Arioz B I,Tastan B,Tarakcioglu E,et al. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway[J]. Front Immunol,2019,10:1511. doi: 10.3389/fimmu.2019.01511 [34] Li X,Luo Z,Gu C,et al. Common variants on 6q16.2,12q24.31 and 16p13.3 are associated with major depressive disorder[J]. Neuropsychopharmacology,2018,43(10):2146-2153. doi: 10.1038/s41386-018-0078-9 [35] Surprenant A,Rassendren F,Kawashima E,et al. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7)[J]. Science,1996,272(5262):735-738. doi: 10.1126/science.272.5262.735 [36] Yang Y,Xing M J,Li Y,et al. Reduced NLRP3 inflammasome expression in the brain is associated with stress resilience[J]. Psychoneuroendocrinology,2021,128:105211. doi: 10.1016/j.psyneuen.2021.105211 [37] Zunszain P A,Anacker C,Cattaneo A,et al. Interleukin-1β:a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis[J]. Neuropsychopharmacology,2012,37(4):939-949. doi: 10.1038/npp.2011.277 [38] Su W J,Zhang T,Jiang C L,et al. Clemastine Alleviates Depressive-Like Behavior Through Reversing the Imbalance of Microglia-Related Pro-inflammatory State in Mouse Hippocampus[J]. Front Cell Neurosci,2018,12:412. [39] Basso A M,Bratcher N A,Harris R R,et al. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety:relevance for neuropsychiatric disorders[J]. Behav Brain Res,2009,198(1):83-90. doi: 10.1016/j.bbr.2008.10.018 [40] Tan S,Wang Y,Chen K,et al. Ketamine Alleviates Depressive-Like Behaviors via Down-Regulating Inflammatory Cytokines Induced by Chronic Restraint Stress in Mice[J]. Biol Pharm Bull,2017,40(8):1260-1267. doi: 10.1248/bpb.b17-00131 -






 下载:
下载: