Cohort Study on the Influence of Opportunistic Infectious Pathogens on the Distribution of Peripheral Blood T Lymphocyte Subsets in Newly Acquired AIDS Patients During Antiretroviral Therapy
-
摘要:
目的 探讨机会性感染病原体对新发艾滋病患者高效抗逆转录病毒治疗过程中外周血T淋巴细胞亚群分布的影响。 方法 收集2019年1月1日至2020年6月30日昆明市第三人民医院感染一科收治的初次确诊且未经抗病毒治疗的艾滋病患者220例,经临床诊断,220例中艾滋病合并结核分枝杆菌33例,艾滋病合并丙型肝炎病毒30例,艾滋病合并马尔尼菲篮状菌31例,其余病例中艾滋病合并1种病原体45例,艾滋病合并2种病原体30例,艾滋病合并3种及以上病原体51例,治疗方案为富马酸替诺福韦二吡呋酯片 + 拉米夫定 + 依非韦伦(TDF + 3TC + EFV),分别于治疗前、治疗3、6、12个月采集患者抗凝血样本,采用流式细胞术检测患者外周血T淋巴细胞亚群,对比分析各组患者抗病毒治疗过程中外周血T淋巴细胞亚群分布情况。 结果 艾滋病合并结核分枝杆菌(AIDS/TB)、艾滋病合并丙型肝炎病毒(AIDS/HCV)、艾滋病合并马尔尼菲篮状菌(AIDS/TM)3组患者在治疗3个月后CD3+、CD8+和CD4+T淋巴细胞计数都明显升高,差异有统计学意义(P < 0.05),除了AIDS/TM组治疗6个月后CD4+T淋巴细胞计数再次明显升高,其余2组3项指标随后治疗过程中无明显变化,差异无统计学意义(P > 0.05);治疗1a后3组患者的CD3+和CD8+T淋巴细胞计数无明显差异,差异无统计学意义(P > 0.05);而AIDS/HCV组CD4+T淋巴细胞计数要高于其它2个组,差异有统计学意义(P < 0.05)。艾滋病合并1种病原体(AIDS/1)组抗病毒治疗3个月后CD3+和CD8+T淋巴细胞计数明显升高,差异有统计学意义(P < 0.05),整个治疗过程中CD4+T淋巴细胞计数无明显变化,差异无统计学意义(P > 0.05);艾滋病合并2种病原体(AIDS/2)组治疗6个月后CD3+及治疗3个月后CD8+T淋巴细胞计数明显升高,差异有统计学意义(P < 0.05);艾滋病合并3种及以上病原体(AIDS/≥3)组治疗6个月后,CD3+和CD8+T淋巴细胞计数才明显升高;AIDS/2和AIDS/≥3组在治疗3、6个月后CD4+T淋巴细胞计数明显升高,差异有统计学意义(P < 0.05)。治疗1年后3组患者的CD3+和CD8+T淋巴细胞计数无明显差异,差异无统计学意义(P > 0.05);而AIDS/1组CD4+T淋巴细胞计数要高于其它2个组,差异有统计学意义(P < 0.05)。6组患者1a的治疗过程中CD4+/CD8+比值都 < 1。 结论 规范的高效抗逆转录病毒治疗后,艾滋病患者外周血CD3+和CD8+T淋巴细胞计数都能恢复到同等水平与机会性感染病原体的种类和数量无关,而艾滋病患者治疗前T淋巴细胞亚群分布水平的高低和机会性感染病原体的种类及数量会影响治疗过程中CD4+T淋巴细胞计数的恢复速度和水平。高效抗逆转录病毒治疗前3个月是评估治疗效果好坏的关键期,而CD4/CD8不是一个理想的治疗效果评估指标。 -
关键词:
- 艾滋病 /
- 机会性感染 /
- T淋巴细胞亚群 /
- 高效抗逆转录病毒治疗
Abstract:Objective To investigate the influence of opportunistic infectious pathogens on the distribution of peripheral blood T lymphocyte subsets in newly acquired AIDS patients during efficient antiretroviral therapy. Methods A total of 220 AIDS patients who were first diagnosed and not performed with antiviral treatment in Infectious Disease Department I of Kunming Third People's Hospital from January 1, 2019 to June 30, 2020 were collected. According to clinical diagnosis, out of 220 patients, 33 patients had AIDS combined with mycobacterium tuberculosis, 30 patients had AIDS combined with hepatitis C virus, 31 patients had AIDS with Talaromyces marneffei, 45 patients had AIDS combined with 1 pathogens, 30 patients had AIDS with 2 pathogens, and 51 patients had AIDS with 3 or more pathogens. The treatment regimen was Tenofovir disoproxil fumarate tablets + lamivudine + EFV (TDF + 3TC + EFV). Anticoagulant samples were collected before treatment, 3, 6 and 12 months after treatment. The peripheral blood T lymphocyte subsets were detected by flow cytometry. The distribution of peripheral blood T lymphocyte subsets in each group during antiviral treatment was compared and analyzed. Results The counts of CD3+, CD8+ and CD4+T lymphocyte in 3 groups of patients including the patients with AIDS combined with mycobacterium tuberculosis (AIDS/TB), the patients with AIDS combined with hepatitis c virus (AIDS/HCV) and the patients with AIDS combined with Talaromyces marneffei (AIDS/TM) were significantly increased after 3 months treatment, and the differences were of statistical significance (P < 0.05). As for AIDS/TM group, the CD4+T lymphocyte count increased significantly after 6 months of treatment; As for the other two groups, there was no obvious change of 3 indexes in the subsequent treatment, and the difference was statistically significant. (P > 0.05). There was no significant difference in the count of CD3+ and CD8 + T lymphocytes in the three groups after 1 year of treatment, and the difference was of no statistical significance (P > 0.05); The CD4 + T lymphocyte count in AIDS/HCV group was higher than that of the other two groups, and the difference was statistically significant (P < 0.05). The CD3+ and CD8+T lymphocyte counts increased significantly after 3 months of antiviral treatment in the group of AIDS with 1 pathogen (AIDS/1), and the difference was statistically significant (P < 0.05). There was no significant change in CD4+T lymphocyte count during the whole treatment period, and the difference was no statistically significant (P > 0.05). The CD8+T lymphocyte counts after treatment for 6 months and the CD3+ lymphocyte counts after treatment for 3 months in the group of AIDS with 2 pathogens increased significantly, and the difference was statistically significant (P < 0.05). The CD3+ and CD8+T lymphocyte counts increased significantly after 6 months of treatment in the group of AIDS with 3 or more pathogens (AIDS/≥3); The CD4+T lymphocyte count of AIDS/2 and AIDS/≥3 groups increased significantly after 3 and 6 months of treatment, and the difference was statistically significant (P < 0.05). After one year of treatment, there was no significant difference in CD3+ and CD8+T lymphocyte counts among the three groups, and the difference was no statistically significant (P > 0.05); The CD4+T lymphocyte count of AIDS/1 group was higher than that of the other two groups, and the difference was statistically significant (P < 0.05). The ratio of CD4+/CD8+ in 6 groups was less than 1. Conclusions After the standardized efficient antiretroviral therapy, the counts of CD3+ and CD8+T lymphocytes in the peripheral blood of AIDS patients can be restored to the same level, regardless of the type and quantity of opportunistic pathogens. The distribution level of T lymphocyte subsets in the AIDS patients before and after treatment and the type and quantity of opportunistic pathogens will affect the recovery rate and level of CD4+T lymphocyte count during treatment. Three months before the efficient antiretroviral treatment is the key period to evaluate the therapeutic effect, while CD4/CD8 is not an ideal evaluation index. -
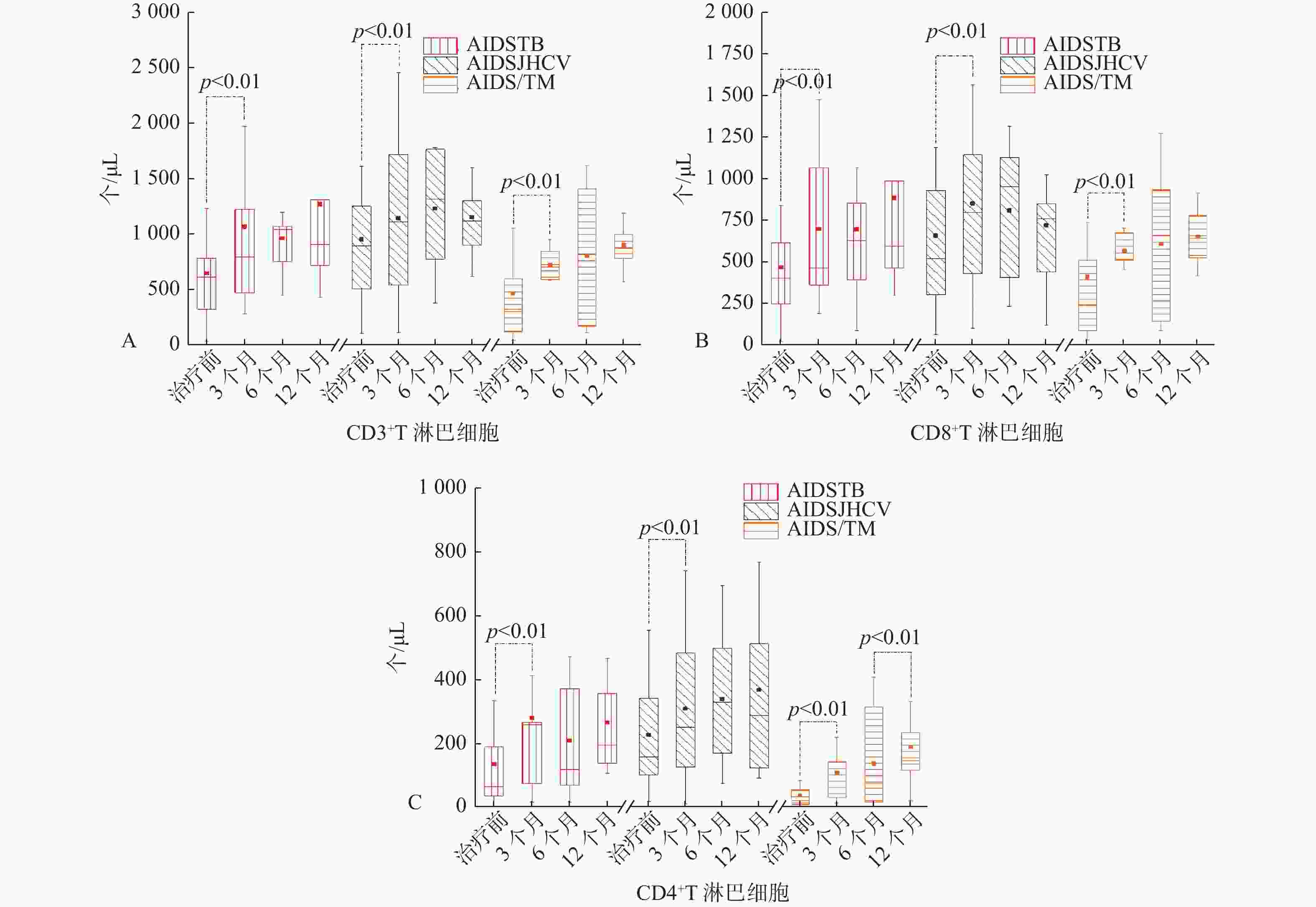
表 1 AIDS/TB 、AIDS/HCV 、AIDS/TM 三组患者抗病毒治疗过程中T淋巴细胞亚群分布特征[(
$ \bar x \pm s $ ,M(P25,P75)]Table 1. The distribution characteristics of T lymphocyte subsets in peripheral blood of patients in 3groups during antiviral therapy [
$ \bar x \pm s $ ,M(P25,P75)]指标 CD3+
(个/μL)CD3+CD8+
(个/μL)CD3+CD4+
(个/μL)CD3+/
淋巴细胞(%)CD3+CD8+/
淋巴细胞(%)CD3+CD4+/
淋巴细胞(%)CD4+/
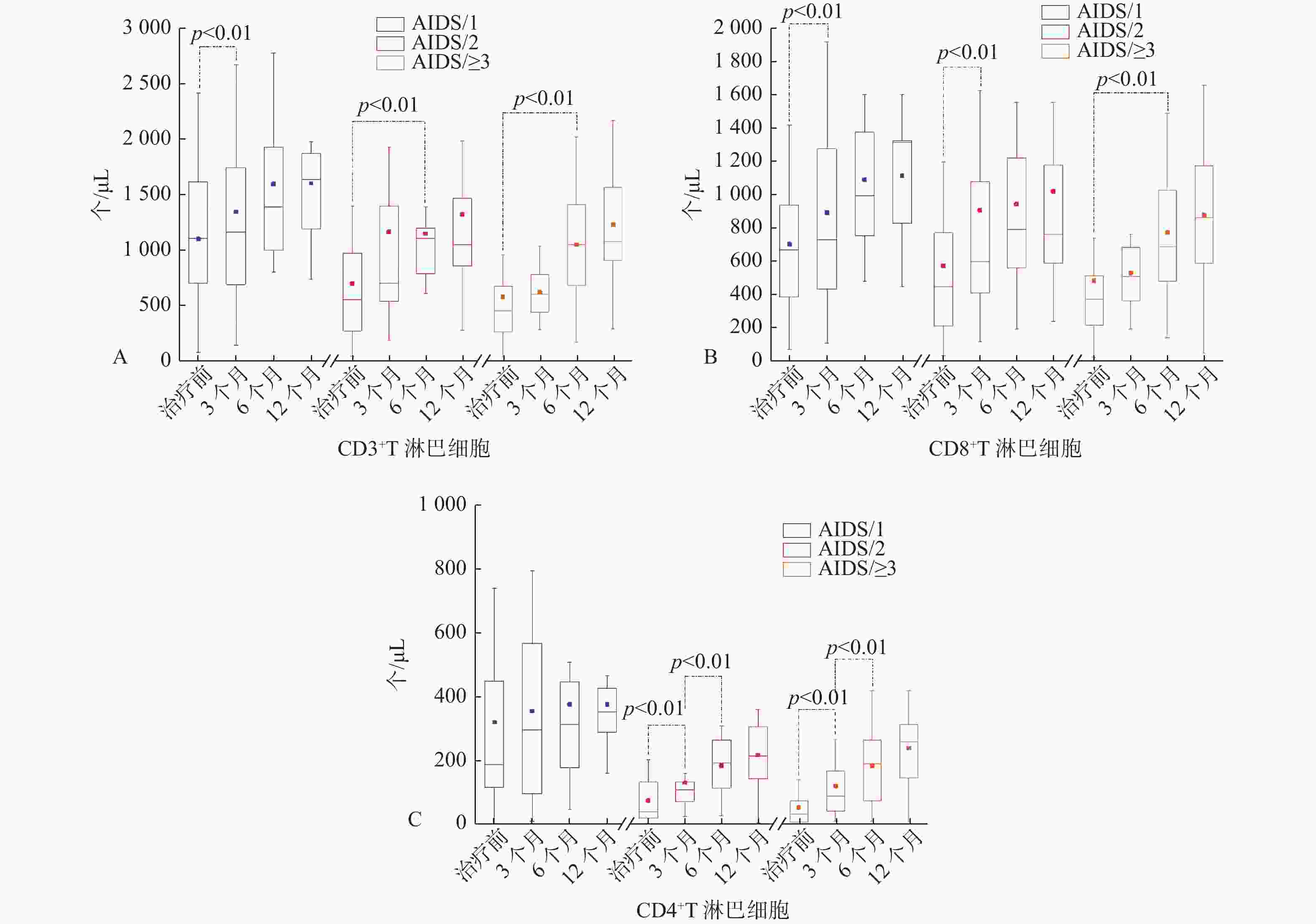
CD8+治疗前 AIDS/TB 615.1(309.6,854.5) 471.2 ± 291.3 65.9(35.0,195.1) 65.9 ± 12.9 49.9 ± 15.1 8.3(4.6,16.6) 0.3(0.1,0.5) AIDS/HCV 896.5(498.2,1273.9) 661.6 ± 582.1 158.3(94.1,346.5) 69.1 ± 12.3 47.1 ± 15.0 13.4(9.3,21.3) 0.3(0.1,0.6) AIDS/TM 326.6(114.1,624.8) 242.4(86.1,571.6) 21.85(7.1,56.8) 59.8 ± 16.1 52.4(34.8,61.2) 5.2 ± 3.2 0.1(0.1,0.2) 治疗3个月 AIDS/TB 800.4(472.9,1228.2) 663.7(362.4,1068.1) 259.9(75.4,267.2) 71.8 ± 10.0 49.6 ± 11.8 16.7 ± 10.8 0.4 ± 0.1 AIDS/HCV 1146.2 ± 750.4 855.1 ± 544.8 309.5 ± 234.2 76.8(69.5,79.9) 49.1 ± 17.4 18.5 ± 12.8 0.3(0.2,0.8) AIDS/TM 706.5(592.0,849.3) 597.4(512.4,676.7) 82.2(30.5,142.8) 76.1(71.3,82.4) 56.6 ± 16.2 11.8 ± 7.5 0.2(0.1,0.3) 治疗6个月 AIDS/TB 958.2(752.8,1075.4) 671.1 ± 409.3 209.5(70.9,372.4) 69.9 ± 11.0 48.1 ± 18.1 15.3 ± 10.7 0.2(0.1,0.6) AIDS/HCV 1236.1 ± 499.5 810.9 ± 384.3 340.0 ± 187.8 69.8 ± 10.4 44.7 ± 10.7 19.9 ± 10.3 0.4(0.3,0.7) AIDS/TM 806.2 ± 559.3 611.9 ± 404.2 74.5(15.3,316.6) 65.9 ± 17.0 51.2 ± 13.4 9.4 ± 6.7 0.2(0.1,0.3) 治疗12个月 AIDS/TB 900.7(755.9,1315.8) 579.4(462.1,987.7) 196.1(139.9,372.7) 67.9 ± 12.1 47.4 ± 12.7 15.3 ± 4.9 0.3(0.2,0.6) AIDS/HCV 1157.7 ± 595.3 722.4 ± 358.4 368.7 ± 269.3 71.8(61.7,74.9) 42.9 ± 10.0 21.5 ± 9.9 0.5 ± 0.3 AIDS/TM 905.8.7 ± 259.3 652.9 ± 167.1 189.2 ± 117.4 74.9 ± 8.6 53.3(47.6,63.0) 14.7 ± 6.3 0.3(0.2,0.4) 备注:AIDS/TB 33例;AIDS/HCV 30例;AIDS/TM 31例。 表 2 AIDS/1、AIDS/2、AIDS/≥3三组患者抗病毒治疗过程中T淋巴细胞亚群分布特征[
$ \bar x \pm s $ ,M(P25,P75)]Table 2. the distribution characteristics of T lymphocyte subsets in peripheral blood of patients in 3groups during antiviral therapy[
$ \bar x \pm s $ ,M(P25,P75)]指标 CD3+
(个/μL)CD3+CD8+
(个/μL)CD3+CD4+
(个/μL)CD3+/
淋巴细胞(%)CD3+CD8+/
淋巴细胞(%)CD3+CD4+/
淋巴细胞(%)CD4+/
CD8+治疗前 AIDS/1 1097.1 ± 568.0 701.9 ± 387.5 315.6 ± 256.3 72.9(65.6,78.8) 47.1 ± 14.9 18.9 ± 12.2 0.4(0.2,0.7) AIDS/2 693.7(516.3,916.4) 447.6(210.7,774.1) 42.8(21.9,135.6) 61.9 ± 16.3 50.5 ± 14.5 7.7 ± 5.0 0.1(0.1,0.2) AIDS/≥3 451.4(253.3,672.1) 372.9(211.6,525.6) 35.0(11.6,80.2) 66.5 ± 13.2 55.1 ± 12.1 5.8(3.1,8.6) 0.1(0.1,0.1) 治疗3个月 AIDS/1 1339.8 ± 832.2 892.5 ± 585.4 357.6 ± 303.2 79.1(66.6,80.9) 49.8 ± 16.4 18.6 ± 13.1 0.3(0.2,0.7) AIDS/2 811.1(518.1,1392.9) 596.8(407.6,1080.0) 110.5(76.1,136.7) 69.4 ± 12.6 54.7 ± 13.4 10.5 ± 6.7 0.2(0.1,0.3) AIDS/≥3 596.5(437.9,777.6) 529.3 ± 239.9 91.7(44.7,170.0) 72.5 ± 4.8 56.8(50.0,63.7) 12.9 ± 7.1 0.2(0.1,0.4) 治疗6个月 AIDS/1 1590.6 ± 707.3 1091.4 ± 421.6 377.7 ± 292.6 74.1 ± 9.0 52.3 ± 10.0 15.9 ± 7.0 0.3(0.2,0.4) AIDS/2 1100.0(780.3,1385.0) 789.0(560.3,1221.2) 186.3 ± 85.5 67.0 ± 11.5 50.9 ± 14.1 11.7 ± 6.6 0.3 ± 0.2 AIDS/≥3 1042.7 ± 483.7 772.9 ± 377.5 185.1 ± 112.9 68.8 ± 10.4 51.0 ± 10.7 12.5 ± 7.0 0.2(0.1,0.4) 治疗12个月 AIDS/1 1635.0(1153.9,1868.6) 1115.3(827.6,1338.7) 354.7(244.3,428.5) 75.5(72.2,80.6) 53.7 ± 8.6 16.8 ± 5.3 0.3(0.2,0.5) AIDS/2 1045.2(838.5,1549.2) 765.4(588.2,1247.8) 219.3 ± 101.4 70.6(53.8,75.0) 50.2 ± 12.2 11.9 ± 5.8 0.3(0.1,0.4) AIDS/≥3 1223.6 ± 465.5 877.9 ± 376.1 241.5 ± 105.9 69.4(60.7,78.6) 49.0 ± 12.5 13.1 ± 5.1 0.3(0.2,0.4) 备注:AIDS/1 45例;AIDS/2 30例;AIDS/≥3 51例。 -
[1] Ghosn J,Taiwo B,Seedat S,et al. HIV[J]. Lancet,2018,392(10148):685-697. doi: 10.1016/S0140-6736(18)31311-4 [2] Lu D Y,Wu H Y,Yarla N S,et al. HAART in HIV/AIDS treatments:Future trends[J]. Infect Disord Drug Targets,2018,18(1):15-22. doi: 10.2174/1871526517666170505122800 [3] Cao W,Hsieh E,Li T. Optimizing treatment for adults with HIV/AIDS in China:Successes over two decades and remaining challenges[J]. Curr HIV/AIDS Rep,2020,17(1):26-34. doi: 10.1007/s11904-019-00478-x [4] Shmakova A,Germini D,Vassetzky Y. HIV-1,HAART and cancer:A complex relationship[J]. Int J Cancer,2020,146(10):2666-2679. doi: 10.1002/ijc.32730 [5] 郑艳,王志勇,陈橙,等. HIV感染者/AIDS患者高效抗逆转录病毒治疗感知效果影响因素分析[J]. 重庆医科大学学报,2021,46(4):493-498. [6] WS293-2019, 艾滋病和艾滋病病毒感染诊断[S]. 北京: 中国标准出版社, 2019. [7] 中华医学会感染病学分会艾滋病丙型肝炎学组,中国疾病预防与控制中心. 中国艾滋病诊疗指南(2018版)[J]. 中华传染病杂志,2018,36(12):705-724. doi: 10.3760/cma.j.issn.1000-6680.2018.12.001 [8] Yang H Y,Beymer M R,Suen S C. Chronic disease onset among people living with HIV and AIDS in a large private insurance claims dataset[J]. Sci Rep,2019,9(1):18514. doi: 10.1038/s41598-019-54969-3 [9] 中华人民共和国国家统计局. 中国统计年鉴[M]. 北京: 中国统计出版社, 2019: 23-24. [10] 杜波,马玉霞,田晓东,等. 阜新市高效抗反转录病毒治疗的HIV/AIDS病人基因型耐药情况及影响因素[J]. 中国艾滋病性病,2020,26(8):814-818. [11] 周超,陈宗良,张维,等. 重庆市艾滋病抗病毒治疗新入组病人治疗效果及其影响因素分析[J]. 现代预防医学,2019,46(17):3091-3095. [12] Jia X C,Xia Z H,Shi N,et al. The factors associated with natural disease progression from HIV to AIDS in the absence of ART,a propensity score matching analysis[J]. Epidemiol Infect,2020,24(5):148-157. [13] Li Y Y,Yang S H,Wang R R,et al. Effects of CD4 cell count and antiretroviral therapy on mucocutaneous manifestations among HIV/AIDS patients in Yunnan,China[J]. Int J Dermatol,2020,59(3):308-313. doi: 10.1111/ijd.14725 [14] Yang X,Su B,Zhang X,et al. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy:Challenges of immunological non-responders[J]. J Leukoc Biol,2020,107(4):597-612. doi: 10.1002/JLB.4MR1019-189R [15] 黄山,吕松琴,徐丽萍,等. 2015-2019年云南地区HIV感染者/AIDS患者合并感染病原微生物特征分析[J]. 临床检验杂志,2021,39(2):151-153. -





 下载:
下载: