Clinical Features of Adult HPS and the Correlation between Cytokine Levels and Prognosis
-
摘要:
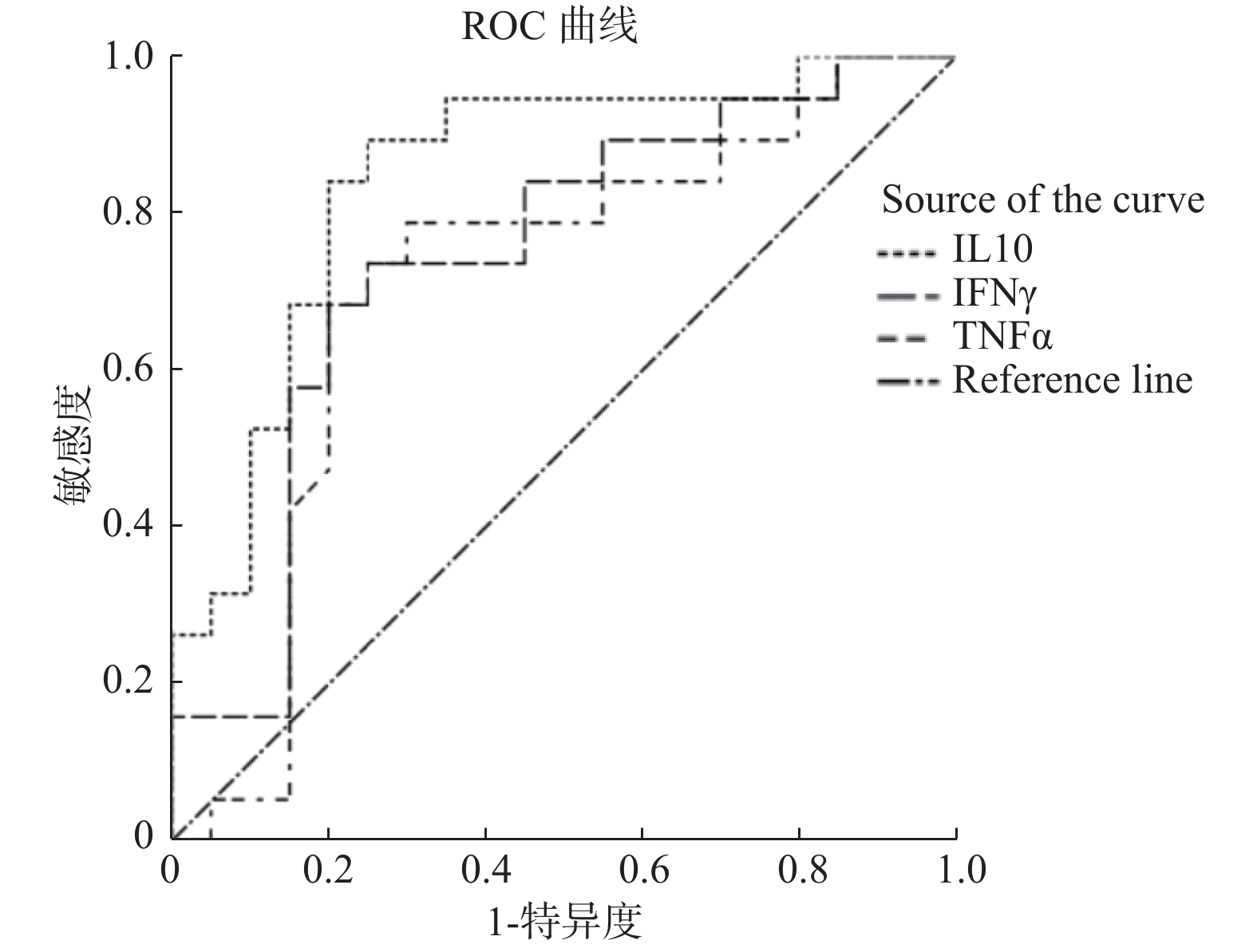
目的 分析成人噬血细胞综合征(hemophagocytic syndrome,HPS)患者临床特征及多种细胞因子水平与预后相关性。 方法 收集19例成人噬血细胞综合征患者资料,分析其临床特征、实验室检查,并收集其血液样本进行12项细胞因子水平检测,分析其与预后的相关性。 结果 HPS患者细胞因子IL-1β、IL-2、IL-4、IL-8、IL-10、IL-6、IFN-γ、TNF-α的水平高于非HPS非感染患者组(P < 0.05);HPS患者细胞因子IL-4、IL-5、IL-10、IFN-γ、TNF-α的水平高于非HPS感染患者组(P < 0.05),IL-10、IFN-γ、TNF-α3种细胞因子ROC曲线示其AUC在0.7~0.9之间,用于诊断HPS具有一定的准确性。HPS死亡组患者发病时Fib明显低于存活组(P < 0.05),而HPS死亡组患者发病时年龄、性别、临床特征(发热、肝脾、淋巴结肿大)及实验室检查(ALT、AST、TBIL、ALB、Fer、TG、ACN、HGB、PLT)与存活组,差异无统计学意义(P > 0.05),而HPS死亡组患者发病时,IL-1β、IL-8、TNF-α明显高于存活组(P < 0.05)。 结论 HPS可出现多种细胞因子水平升高,其中IL-10、IFN-γ、TNF-α明显升高可能对其诊断具有一定意义,Fib明显下降及IL-1β、IL-8、TNF-α明显升高可能提示预后不良。 Abstract:Objective To analyze the clinical features of adult hemophagocytic syndrome (HPS) and the correlation between the levels of various cytokines and prognosis. Methods The data of 19 adult patients with hemophagocytic syndrome were collected, and the clinical features and laboratory tests were analyzed. The blood samples were collected for 12 cytokine levels detection, and the correlation between the levels and prognosis was analyzed. Results The levels of cytokines IL-1β, IL-2, IL-4, IL-8, IL-10, IL-6, IFN-γand TNF-α in HPS patients were higher than those in non-HPS non-infected patients (P < 0.05). The levels of IL-4, IL-5, IL-10, IFN-γ and TNF-α in HPS patients were higher than those in non-HPS patients (P < 0.05), the ROC curve of IL-10, IFN-γ and TNF-α showed that the AUC of IL-10, IFN-γ and TNF-α was between 0.7 and 0.9, indicating accuracy in the diagnosis of HPS. Fib was significantly lower than that in the survival group (P < 0.05), while there were no significant differences in age, sex, clinical features (fever, liver, spleen, lymphadenopathy) and laboratory tests (ALT, AST, TBIL, ALB, Fer, TG, ACN, HGB, PLT) between the HPS death group and the survival group (P > 0.05), and the levels of IL-1β, IL-8 and TNF-α in HPS death group were significantly higher than those in survival group (P < 0.05). Conclusion HPS may present increased levels of various cytokines, among which IL-10, IFN-γ and TNF-α may be significant for the diagnosis of HPS. The significant decrease of Fib and the significant increase of IL-1β, IL-8 and TNF-α may indicate a poor prognosis. -
Key words:
- Hemophagocytic syndrome /
- Clinical features /
- Cytokines
-
表 1 HPS患者死亡与存活组年龄及实验室检查比较 M(Q1,Q3)(1)
Table 1. Comparison of age and laboratory examination between death and survival groups of patients with HPS M(Q1,Q3)(1)
组别 年龄
(岁)ANC
(×109/L)HGB
(g/L)PLT
(×109/L)Fib
(g/L)TG
(mmol/L)HPS患者
死亡组42
(22.5,55.5)0.97
(0.78,1.93)80
(64,105)30
(21,73)0.97
(0.78,1.93)3.49
(2.22,4.26)HPS患者
存活组37.5
(18.5,53.75)1.35
(0.62,2.75)83.5
(63.25,110.25)57.5
(32.25,165.25)2.6
(1.80,4.95)3.66
(3.0,4.54)Z −0.351 −0.439 −0.044 −1.097 −2.369 −0.877 P 0.725 0.661 0.965 0.273 0.018* 0.380 Ball Test 0.019 0.105 0.033 0.091 0.260 0.086 P 0.990 0.310 0.960 0.410 0.040* 0.520 ACN:中性粒细胞计数;HGB:血红蛋白,PLT:血小板计数;Fib:纤维蛋白原;TG:甘油三酯;Fer:铁蛋白;ALB:白蛋白;AST:谷草转氨酶;ALT:谷丙转氨酶;TBIL:总胆红素;LDH:乳酸脱氢酶。*P < 0.05。 表 1 HPS患者死亡与存活组年龄及实验室检查比较 M(Q1,Q3)(2)
Table 1. Comparison of age and laboratory examination between death and survival groups of patients with HPS M(Q1,Q3)(2)
组别 Fer
(μg/L)AST
(IU/L)ALT
(IU/L)ALB
(g/L)TBIL
(μmol/L)LDH
(IU/L)HPS患者
死亡组2026
(1500,14887)167
(48.25,343.05)106.5
(50.05,264.05)24.2
(23.75,26.7)30.80
(19.95,136)1134
(363,2519)HPS患者
存活组2050.25
(1351.75,4862.25)82.55
(25.35,236.55)65.15
(27.83,106.1)25.2
(21.45,28.7)20.6
(7.47,77.83)414
(305.25,1689)Z −0.796 −1.140 −1.403 0.000 −1.141 −1.052 P 0.426 0.254 0.161 1.000 0.254 0.293 Ball Test 0.090 0.073 0.122 0.046 0.083 0.138 P 0.460 0.600 0.290 0.950 0.540 0.230 ACN:中性粒细胞计数;HGB:血红蛋白,PLT:血小板计数;Fib:纤维蛋白原;TG:甘油三酯;Fer:铁蛋白;ALB:白蛋白;AST:谷草转氨酶;ALT:谷丙转氨酶;TBIL:总胆红素;LDH:乳酸脱氢酶。 表 2 HPS患者与非HPS未感染患者12项细胞因子水平比较 M(Q1,Q3)(1)
Table 2. Comparison of 12 cytokine levels in HPS patients and non-HPS uninfected patients M(Q1,Q3)(1)
组别 IL-1β
(pg/mL)IL-2
(pg/mL)IL-4
(pg/mL)IL-5
(pg/mL)IL-8
(pg/mL)IL-10
(pg/mL)HPS患者 3.32
(0.63,31.67)1.46
(0.73,3.02)0.96
(0.65,1.68)1.42
(1.00,2.08)20.24
(4.4,130.94)111.14
(11.09,1009.5)非HPS
未感染患者0.47
(0.34,0.61)0.4
(0.26,0.70)0.49
(0.38,0.59)0.57
(0.34,2.68)0.51
(0.26,3.59)0.78
(0.54,1.08)Z −3.374 −2.72 −3.374 −1.307 −3.768 −4.923 P 0.001* 0.007* 0.001* 0.191 0.000* 0.000* Ball Test 0.276 0.195 0.299 0.032 0.252 0.469 P 0.010* 0.010* 0.010* 0.730 0.010* 0.010* *P < 0.05。 表 2 HPS患者与非HPS未感染患者12项细胞因子水平比较 M(Q1,Q3)(2)
Table 2. Comparison of 12 cytokine levels in HPS patients and non-HPS uninfected patients M(Q1,Q3)(2)
组别 IL-12P70
(pg/mL)IL-6
(pg/mL)IL-17
(pg/mL)IFN-α
(pg/mL)IFN-γ
(pg/mL)TNF-α
(pg/mL)HPS患者 0.65
(0.36,1.25)37.5
(11.82,70.63)0.91
(0.45,1.29)0.65
(0.36,1.25)23.1
(4.24,57.48)3.6
(1.4,6.87)非HPS
未感染患者0.745
(0.41,1.00)0.85
(0.31,3.53)0.76
(0.64,1.12)0.64
(0.45,0.97)3.40
(3.01,4.90)0.52
(0.35,1.57)Z −1.033 −4.589 −0.395 −0.167 −2.978 −2.599 P 0.301 0.000* 0.693 0.867 0.003* 0.009* Ball Test 0.038 0.068 0.059 0.042 0.237 0.226 P 0.070 0.070 0.130 0.320 0.010* 0.010* *P < 0.05。 表 3 HPS患者与非HPS感染患者12项细胞因子水平比较 M(Q1,Q3)(1)
Table 3. Comparison of 12 cytokine levels in HPS patients and non-HPS infected patients M(Q1,Q3)(1)
组别 IL-1β
(pg/mL)IL-2
(pg/mL)IL-4
(pg/mL)IL-5
(pg/mL)IL-8
(pg/mL)IL-10
(pg/mL)HPS患者 3.32
(0.63,31.67)1.46
(0.73,3.02)0.96
(0.65,1.68)1.42
(1.00,2.08)20.24
(4.4,130.94)111.14
(11.09,1009.5)非HPS
感染患者1.72
(0.54,8.56)1.04
(0.49,2.25)0.35
(0.18,0.77)1.07
(0.22,20.39)58.29
(20.59,166.42)1.49
(0.87,5.57)Z −0.618 −0.520 −2.852 −2.444 −1.967 −3.709 P 0.536 0.603 0.004* 0.015* 0.49 0.000* Ball Test 0.016 0.026 0.125 0.197 0.063 0.222 P 0.830 0.620 0.030* 0.010* 0.130 0.010* *P < 0.05。 表 3 HPS患者与非HPS感染患者12项细胞因子水平比较 M(Q1,Q3)(2)
Table 3. Comparison of 12 cytokine levels in HPS patients and non-HPS infected patients M(Q1,Q3)(2)
组别 IL-12P70
(pg/mL)IL-6
(pg/mL)IL-17
(pg/mL)IFN-α
(pg/mL)IFN-γ
(pg/mL)TNF-α
(pg/mL)HPS患者 0.65
(0.36,1.25)37.5
(11.82,70.63)0.91
(0.45,1.29)0.65
(0.36,1.25)23.1
(4.24,57.48)3.6
(1.4,6.87)非HPS
感染患者0.71
(0.29,0.97)60.24
(30.09,146.66)1.18
(0.63,1.92)0.86
(0.20,1.89)3.42
(1.66,8.08)1.14
(0.30,1.72)Z −1.055 −1.883 −0.942 −0.225 −2.613 −2.051 P 0.291 0.060 0.346 0.822 0.009* 0.040* Ball Test 0.040 0.036 0.021 0.036 0.117 0.140 P 0.290 0.410 0.730 0.380 0.020* 0.010* *P < 0.05。 表 4 HPS患者死亡与存活组12项细胞因子水平比较 M(Q1,Q3)(1)
Table 4. Comparison of 12 cytokine levels in the HPS patient death and survival group M(Q1,Q3)(1)
组别 IL-1β
(pg/mL)IL-2
(pg/mL)IL-4
(pg/mL)IL-5
(pg/mL)IL-8
(pg/mL)IL-10
(pg/mL)HPS患者
死亡组7.89
(2.61,32.67)1.82
(1.01,10.72)1.04
(0.76,2.14)1.89
(0.95,2.22)30.22
(14.78,139.31)71.00
(11.40,1596.56)HPS患者
存活组0.59
(0.35,1.33)1.05
(0.40,1.85)0.84
(0.39,1.12)1.12
(0.87,1.48)4.95
(2.62,24.38)176.20
(7.43,741.09)Z −2.543 −1.403 −1.052 −1.140 −2.017 −0.263 P 0.011* 0.161 0.293 0.254 0.044* 0.792 Ball Test 0.407 0.102 0.124 0.092 0.248 0.052 P 0.010* 0.450 0.300 0.490 0.007* 0.810 *P < 0.05。 表 4 HPS患者死亡与存活组12项细胞因子水平比较 M(Q1,Q3)(2)
Table 4. Comparison of 12 cytokine levels in the HPS patient death and survival group M(Q1,Q3)(2)
组别 IL-12P70
(pg/mL)IL-6
(pg/mL)IL-17
(pg/mL)IFN-α
(pg/mL)IFN-γ
(pg/mL)TNF-α
(pg/mL)HPS患者
死亡组0.94
(0.46,2.23)33.71
(11.26,74.24)0.86
(0.26,1.27)0.65
(0.39,1.06)23.10
(9.19,247.80)5.47
(2.48,7.74)HPS患者
存活组0.65
(0.28,1.12)40.31
(22.52,73.51)1.31
(0.77,3.33)0.92
(0.20,6.13)15.46
(2.87,56.06)1.02
(0.23,2.33)Z −0.879 −0.351 −1.756 −0.351 −0.877 −2.543 P 0.379 0.726 0.079 0.725 0.380 0.011* Ball Test 0.066 0.158 0.098 0.115 0.074 0.317 P 0.700 0.160 0.400 0.340 0.650 0.010* *P < 0.05。 -
[1] Risdall R J,McKenna R W,Nesbit M E,et al. Virus-associated hemophagocytic syndrome:A benign histiocytic proliferation distinct from malignant histiocytosis[J]. Cancer,1979,44(3):993-1002. doi: 10.1002/1097-0142(197909)44:3<993::AID-CNCR2820440329>3.0.CO;2-5 [2] 张之南, 沈悌. 血液病诊断及疗效标准[M]. 第3版. 北京: 科学出版社, 2007: 254-256. [3] Ramos Casals M,Brito Zerón P,López Guillermo A,et al. Adult haemophagocytic syndrome[J]. Lancet,2014,383(9927):1503-1516. doi: 10.1016/S0140-6736(13)61048-X [4] KumaKura S,Yamaguchi Y,Murakawa Y,et al. Effect of cytokines and hyperthermia on phagocytosis and phosphatidyiserine externliza- tion:Implication for the pathophysiology of hemophagocytic syndrome[J]. Ann Clin Lab Sci,2018,48(3):314-322. [5] Grom A A. Natural killer cell dysfunction: A common pathway in Systemiconset juvenile rheumatoid arthritis, macrophage activation syndrome, and hemophagocytic lymphohistiocytosis[J] Arthritis & Rheumatism, 2004, 50(3): 689-698. [6] Wang,Xueqin,Pan,et al. Conditional distance correlation[J]. J Am Stat Assoc,2015,110(512):1726-1734. doi: 10.1080/01621459.2014.993081 [7] Yoon J H,Park S S,Jeon Y W,et al. Treament outcomes and prognostic factors in adult patients with secondary hemophagocytic lympho- histiocytosis not associated with malignancy[J]. Haematologica,2019,104(2):269-276. doi: 10.3324/haematol.2018.198655 [8] Zhao Y,Lu D,Ma S,et al. Risk factors of early death in adult patients with secondary hemophagocytic lymphohistiocytosis:A single-in- stitution study of 171 Chinese patients[J]. Hematology,2019,24(1):606-612. doi: 10.1080/16078454.2019.1660458 [9] 闫丽娟,王昭. 噬血细胞综合征继发性凝血功能障碍的认识与研究[J]. 临床和实验医学杂志,2012,11(2):142-143. doi: 10.3969/j.issn.1671-4695.2012.02.034 [10] Chen L,Weng H,Li H,et al. Potential killer in the ICU-severe tuberculosis combined with hemophagocytic syndrome:A case series and literature review[J]. Medicine,2017,96(49):1-5. [11] Mqller H J. Soluble CD163[J]. Scand J Clin Lab In-vest,2012,72(1):1-13. doi: 10.3109/00365513.2011.626868 [12] Sieni E,Cetica V,Mastrodicasa E,et al. Familial hemophagocytic lymphohis- tiocytosis:A model for understanding the human machinery of cellular cytotoxicity[J]. Cell Mol Life Sci,2012,69(1):29-40. doi: 10.1007/s00018-011-0835-y [13] Gayden T,Sepulveda F E,Khuong-Quang D A,et al. Author correction:germline HAVCR2 mutations altering TIM-3 characterize subcutaneous panniculitis-like T cell lymphomas with hemophagocytic lymphohistiocytic syndrome[J]. Nat Genet,2019,51(1):196. [14] Hiroi M,Sakaeda Y,Yamaguchi H,et al. Anti-inflammatory cytokine interleukin-4 inhibits inducible nitric oxide synthase gene expression in the mouse macrophage cell line RAW264.7 through the repression of octamer dependent transcription[J]. Mediators of Inflammation,2013,2013(1):1-15. [15] Klementiev B,Enevoldsen M N,Li S,et al. Anti - inflammatory properties of a peptide derived from interleukin-4[J]. Cytokine,2013,64(1):112-121. doi: 10.1016/j.cyto.2013.07.016 [16] Akdis M,Aab A,Altunbulakli C,et al. Interleukins( from IL-1 to IL-38),interferons,transforming growth factor beta,and TNF-alpha:Receptors,functions,and roles in diseases[J]. J Allergy Clin Immunol,2016,138(4):984-1010. doi: 10.1016/j.jaci.2016.06.033 [17] Yoshimura T,Matsushima K,Oppenheim J,et al. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimula ted human blood mononuclear leukocytes:Partial characterization and separation from interleukin 1 ( IL-1)[J]. J Immunol,1987,139(3):788-793. [18] Peiro T,Patel D F,Akthar S,et al. Neutrophils drive alveolar macrophage IL-1β release during respiratory viral infection[J]. Thorax,2018,73(6):546-556. doi: 10.1136/thoraxjnl-2017-210010 -






 下载:
下载: