Clinical Features and Related Prognostic Factors of 88 Cases of Duodenal Adenocarcinoma
-
摘要:
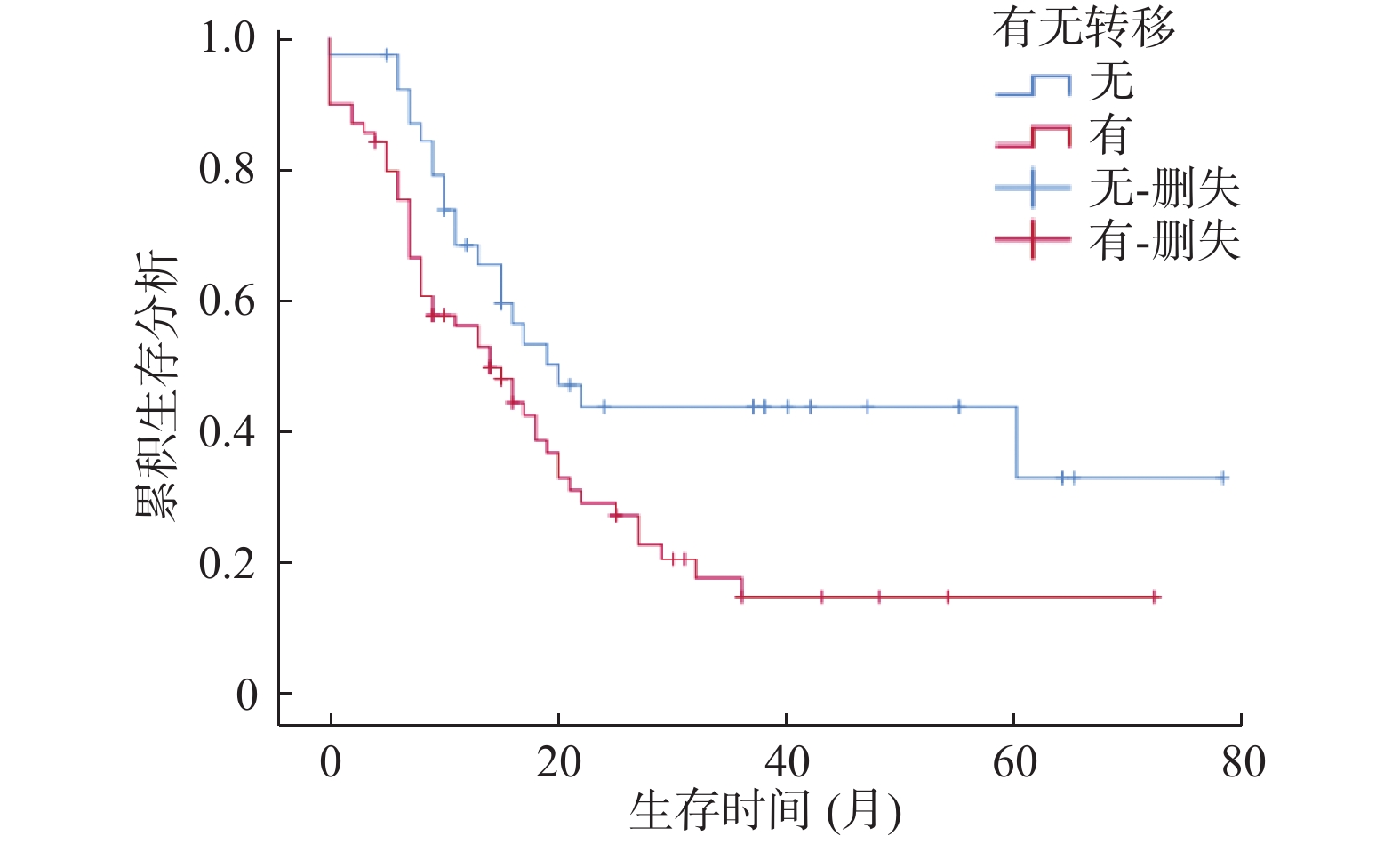
目的 分析十二指肠腺癌患者的临床特征及相关预后因素。 方法 对云南省肿瘤医院2010年1月至2019年12月期间收治的经病理确诊的88例十二指肠腺癌患者进行回顾性研究。采用Kaplan-Meier法评估患者的生存状况,用Log-rank检验进行单因素分析,Cox比例风险回归模型进行多因素分析,从而筛选出影响十二指肠腺癌患者预后的相关危险因素。 结果 88例十二指肠腺癌患者的年龄范围:23~79岁,平均年龄为54.83岁,男女比例:1.44∶1。生存分析提示:十二指肠腺癌患者发生转移、无转移的中位生存时间分别为14个月、19个月,即发生转移较无转移者预后更差(P < 0.05)。单因素分析显示:转移有无、原发灶直径、临床分期、化疗方案、化疗药物种类、手术方式、Hp感染、CEA、CA199、AST/ALT、NKC、PLT、LMR与十二指肠腺癌患者预后有关(P < 0.05)。Cox多因素分析提示:发生转移、Hp(+)、CA199 > 27 U/mL是影响十二指肠腺癌患者预后的独立危险因素(P < 0.05),而根治性手术是十二指肠腺癌患者预后的有益因素(P < 0.05)。 结论 十二指肠腺癌发生转移则预后极差,需早期密切监测,临床上可用有无转移、手术方式、Hp感染、CA199 4个指标预测患者预后。 Abstract:Objective To analyze the clinical characteristics and related prognostic factors of patients with duodenal adenocarcinoma. Methods 88 patients with duodenal adenocarcinoma who were pathologically diagnosed and admitted to Yunnan Cancer Hospital from January 2010 to December 2019 were studied retrospectively. The Kaplan-Meier method was used to evaluate the survival status of patients, the Log-rank test was used for univariate analysis, and the Cox proportional hazard regression model was used for multivariate analysis to screen out the relevant risk factors affecting the prognosis of patients with duodenal adenocarcinoma. Results The age range of 88 patients with duodenal adenocarcinoma: 23-79 years old, with an average age of 54.83 years old, male to female ratio: 1.44∶1. Survival analysis showed that the median survival time of patients with and without metastasis of duodenal adenocarcinoma was 14 months and 19 months respectively, the prognosis of patients with metastasis was worse than those without metastasis (P < 0.05). Univariate analysis showed that the presence or absence of metastasis, primary tumor diameter, clinical stage, chemotherapy regimens, types of chemotherapy drugs, surgical methods, Hp infection, CEA, CA199, AST/ALT, NKC, PLT, LMR were related to the prognosis of duodenal adenocarcinoma patients (P < 0.05). Cox multivariate analysis suggested that metastasis, Hp (+), CA199 > 27 U/mL were the independent risk factors affecting the prognosis of patients with duodenal adenocarcinoma (P < 0.05), and radical surgery was the duodenal Beneficial factors for the prognosis of patients with adenocarcinoma (P < 0.05). Conclusion The prognosis of duodenal adenocarcinoma with metastasis is extremely poor, and early close monitoring is needed. Clinically, the prognosis of patients can be predicted by four indicators of metastasis, surgical method, Hp infection, and CA199. -
Key words:
- Duodenal adenocarcinoma /
- Clinical features /
- Prognosis
-
表 1 临床一般资料与十二指肠腺癌预后的单因素分析
Table 1. Univariate analysis of general clinical data and prognosis of adenocarcinoma of duodenum
变量 分组 n 生存率(%) 中位生存期(月) χ2 P 1 a 3 a 5 a 性别 男 52 92.9 54.5 24.5 16 ± 3.735 3.01 0.083 女 36 76.8 36.6 0.0 22 ± 3.592 年龄(岁) ≤60 61 94.4 61.5 31.3 18 ± 2.784 0.717 0.397 > 60 27 82.8 0.0 0.0 20 ± 1.749 BMI(kg/m2) < 24 67 87.0 62.7 35.8 16 ± 2.293 1.650 0.199 ≥24 21 56.5 0.0 0.0 16 ± 7.273 KPS(分) < 90 33 81.0 6.5 0.0 15 ± 3.464 1.914 0.167 ≥90 55 88.4 65.3 39.6 21 ± 4.021 吸烟史 有 23 61.6 0.0 0.0 15 ± 1.546 0.222 0.637 无 65 85.2 59.9 35.0 17 ± 2.358 饮酒史 有 20 55.6 0.0 0.0 15 ± 1.789 0.032 0.857 无 68 86.0 62.4 38.1 16 ± 2.696 转移 有 57 84.1 52.7 20.2 14 ± 2.603 6.073 0.014* 无 31 73.7 32.6 0.0 19 ± 3.996 原发灶部位 降部 75 95.1 69.9 41.5 18 ± 2.433 1.094 0.295 其他 13 66.5 0.0 0.0 20 ± 1.577 原发灶直径(cm) ≤3 52 81.5 45.8 30.9 19 ± 2.255 8.650 0.003* > 3 36 76.6 39.7 0.0 8 ± 2.022 临床分期 I-II 42 77.6 30.2 0.0 19 ± 2.100 4.353 0.037* II-IV 46 81.3 51.2 0.0 13 ± 3.427 分化程度 低-中 56 85.7 50.3 20.2 16 ± 1.984 0.339 0.560 中-高 32 75.3 34.9 0.0 16 ± 6.030 化疗方案 含氟尿嘧啶类 59 78.2 43.2 0.0 20 ± 2.188 5.236 0.022* 不含氟尿嘧啶类 29 68.2 18.9 0.0 15 ± 2.486 化疗药物种类 单药 25 65.5 12.3 0.0 14 ± 4.451 6.325 0.012* 双药及以上 63 81.3 43.4 37.6 20 ± 4.689 手术方式 胰十二指肠切除术 46 82.6 54.0 31.3 22 ± 4.233 23.903 0.001* 其他手术 8 55.6 33.3 0.0 15 ± 5.963 无 34 75.0 12.2 0.0 9 ± 0.787 粪便隐血 阳性 33 78.1 20.3 0.0 20 ± 3.119 0.037 0.847 阴性 55 86.9 60.2 29.3 20 ± 1.680 HP感染 阳性 38 79.6 30.5 0.0 11 ± 2.222 6.393 0.011* 阴性 50 82.5 50.3 32.7 20 ± 2.347 初诊时症状 1个 33 87.5 38.1 0.0 19 ± 3.493 1.649 0.648 2个 38 83.3 25.9 0.0 16 ± 5.852 3个 12 31.4 0.0 0.0 20 ± 1.829 4个 5 0.0 0.0 0.0 14 ± 2.382 *P < 0.05。 表 2 血液学因素与十二指肠腺癌预后的单因素分析
Table 2. Univariate analysis of hematologic factors and prognosis of adenocarcinoma of duodenum
变量 分组 n 生存率(%) 中位生存期(月) χ2 P 1 a 3 a 5 a CEA(ng/mL) < 3.56 42 79.2 33.5 0.0 20 ± 3.639 9.749 0.002* ≥3.56 46 79.9 47.6 0.0 9 ± 2.833 CA199(U/mL) ≤27 48 79.7 43.5 27.3 20 ± 3.758 12.533 0.001* > 27 40 77.4 41.4 12.9 9 ± 3.008 CA125(U/mL) < 31.99 59 86.1 55.3 26.3 17 ± 2.276 1.539 0.215 ≥31.99 29 71.8 27.2 0.0 13 ± 4.402 ALT(U/L) < 19 27 63.3 0.0 0.0 18 ± 2.733 0.667 0.414 ≥19 61 88.5 58.1 35.4 16 ± 2.642 AST/ALT < 1.15 50 80.3 42.1 26.4 20 ± 2.387 12.081 0.001* ≥1.15 38 76.5 38.6 0.0 8 ± 0.993 NLR < 1.64 18 42.9 0.0 0.0 20 ± 1.512 0.864 0.353 ≥1.64 70 89.7 61.4 44.8 15 ± 2.170 NKC < 19.6% 57 84.6 62.0 30.1 15 ± 1.405 5.993 0.014* ≥19.6% 31 60.0 38.4 0.0 27 ± 21.51 Hb(g/L) < 115 36 69.2 25.0 0.0 18 ± 2.075 0.765 0.382 ≥115 52 87.0 55.5 28.5 15 ± 4.254 PLT(×109/L) < 350 61 85.8 56.9 32.1 18 ± 2.306 8.512 0.004* ≥350 27 69.6 0.0 0.0 9 ± 3.131 白蛋白(g/L) < 40 42 77.1 28.7 0.0 18 ± 2.309 3.028 0.082 ≥40 46 81.6 52.1 0.0 13 ± 4.104 LMR < 5 53 84.8 48.2 30.4 19 ± 2.484 7.248 0.007* ≥5 35 78.5 34.9 0.0 9 ± 3.158 注:NLR = 中性粒细胞/淋巴细胞,NKC = 自然杀伤细胞,Hb = 血红蛋白,LMR = 淋巴细胞/单核细胞。*P < 0.05。 表 3 影响十二指肠腺癌预后的多因素分析
Table 3. Analysis of multiple factors influencing prognosis of duodenal adenocarcinoma
指标 B SE Wald P Exp(B) HR(95%CI) 有无转移 0.716 0.349 4.197 0.041* 2.045 (1.031,4.056) 临床分期 −0.899 0.703 1.634 0.201 0.407 (0.103,1.615) 原发灶直径 1.911 1.345 2.019 0.155 6.757 (0.484,94.257) 化疗方案 0.063 0.425 0.022 0.882 1.065 (0.463,2.450) 化疗药物种类 −0.560 0.364 2.360 0.125 0.571 (0.280,1.167) 手术方式 0.644 0.215 9.000 0.003* 1.904 (1.250,2.899) Hp感染 −0.849 0.372 5.205 0.023* 0.428 (0.206,0.887) CEA −0.204 0.739 0.076 0.782 0.815 (0.192,3.469) CA199 3.346 1.663 4.047 0.044* 28.384 (1.090,739.064) AST/ALT 0.084 1.027 0.007 0.935 1.088 (0.145,8.145) PLT 0.580 0.658 0.777 0.378 1.786 (0.492,6.480) NKC −0.518 0.403 1.648 0.199 0.596 (0.270,1.314) LMR −2.486 1.305 3.631 0.057 0.083 (0.006,1.074) 年龄 1.564 1.588 0.969 0.325 4.776 (0.212,107.445) 吸烟史 0.406 0.608 0.447 0.504 1.502 (0.456,4.941) 饮酒史 0.353 0.607 0.338 0.561 1.423 (0.433,4.680) *P < 0.05。 -
[1] Buchbjerg T,Fristrup C,. Mortensen MB. The incidence and prognosis of true duodenal carcinomas[J]. Surgical Oncology,2015,24(2):110-116. doi: 10.1016/j.suronc.2015.04.004 [2] L ó pez-Dom í nguez J,Busquets J,Secanella L,et al. Duodenal adenocarcinoma:Surgical results of 27 patients treated at a single center[J]. Cir Esp,2019,97(9):523-530. doi: 10.1016/j.ciresp.2019.06.014 [3] Cloyd J M,George E,Visser B C. Duodenal adenocarcinoma:Advances in diagnosis and surgical management[J]. World J Gastrointest Surg,2016,8(3):212-221. doi: 10.4240/wjgs.v8.i3.212 [4] Beane J D,Griffin K F,Levy E B,et al. Duodenal ischemia and upper GI bleeding are dose-limiting toxicities of 24h continuous intra-arterial pancreatic perfusion of gemcitabine following vascular isolation of the pancreatic head:Early results from the Regional Chemotherapy in Locally Advanced Pancreatic Cancer (RECLAP) study[J]. Invest New Drugs,2015,33(1):109-118. doi: 10.1007/s10637-014-0157-7 [5] Burasakarn P,Higuchi R,Nunobe S,et al. Limited resection vs. pancreaticoduodenectomy for primary duodenal adenocarcinoma:A systematic review and meta-analysis[J]. International Journal of Clinical Oncology,2021,26(3):450-460. doi: 10.1007/s10147-020-01840-5 [6] Augustin T,Moslim M A,Cengiz T B,et al. Survival outcomes after surgical management of sporadic or familial adenomatous polyposis associated duodenal cancer[J]. J Surg Oncol,2020,122(6):1132-1144. doi: 10.1002/jso.26131 [7] Solaini L,Jamieson N B,Metcalfe M,et al. Outcome after surgical resection for duodenal adenocarcinoma in the UK[J]. British Journal of Surgery,2015,102(6):676-681. doi: 10.1002/bjs.9791 [8] Poultsides G A,Huang L C,Cameron J L,et al. Duodenal adenocarcinoma:Clinicopathologic analysis and implications for treatment[J]. Ann Surg Oncol,2012,19(6):1928-1935. doi: 10.1245/s10434-011-2168-3 [9] Kakushima N,Kanemoto H,Tanaka M,et al. Treatment for superficial non-ampullary duodenal epithelial tumors[J]. World J Gastroenterol,2014,20(35):12501-12508. doi: 10.3748/wjg.v20.i35.12501 [10] Saito H,Takaya S,Osaki T,et al. Increased apoptosis and elevated Fas expression in circulating natural killer cells in gastric cancer patients[J]. Gastric Cancer,2013,16(4):473-479. doi: 10.1007/s10120-012-0210-1 [11] Hagihara S,Shimizu T,Inoue Y,et al. A complete response to capecitabine and oxaliplatin chemotherapy in primary duodenal carcinoma with liver and nodal metastases:A case report[J]. Surg Case Rep,2018,4(1):125. doi: 10.1186/s40792-018-0532-2 [12] Horimatsu T,Nakayama N,Moriwaki T,et al. A phase II study of 5-fluorouracil/L-leucovorin/oxaliplatin (mFOLFOX6) in Japanese patients with metastatic or unresectable small bowel adenocarcinoma[J]. Int J Clin Oncol,2017,22(5):905-912. doi: 10.1007/s10147-017-1138-6 [13] Aydin D,Sendur M A,Kefeli U,et al. Evaluation of bevacizumab in advanced small bowel adenocarcinoma[J]. Clin Colorectal Cancer,2017,16(1):78-83. doi: 10.1016/j.clcc.2016.04.013 [14] Hirashita T,Ohta M,Tada K,et al. Prognostic factors of non-ampullary duodenal adenocarcinoma[J]. Jpn J Clin Oncol,2018,48(8):743-747. doi: 10.1093/jjco/hyy086 [15] Tian J,Liu J,Guo C,et al. Prognostic factors and treatment outcomes in patients with non-ampullary small bowel adenocarcinoma:Long-term analysis[J]. Medicine,2019,98(17):15381. doi: 10.1097/MD.0000000000015381 [16] Mann K,Gilbert T,Cicconi S,et al. Tumour stage and resection margin status are independent survival factors following partial pancreatoduodenectomy for duodenal adenocarcinoma[J]. Langenbecks Arch Surg,2019,404(4):439-449. doi: 10.1007/s00423-019-01779-w [17] Elberg Dengsø K,Tjørnhøj-Thomsen T,Oksbjerg Dalton S,et al. It’ s all about the CA-19-9. A longitudinal qualitative study of patients’ experiences and perspectives on follow-up after curative surgery for cancer in the pancreas,duodenum or bile-duct[J]. Acta Oncol,2019,58(5):642-649. doi: 10.1080/0284186X.2018.1562212 [18] Wang J,Liu X. Correlation analysis between helicobacter pylori infection status and tumor clinical pathology as well as prognosis of gastric cancer patients[J]. Iran J Public Health,2018,47(10):1529-1536. [19] Liu L P,Sheng X P,Shuai T K,et al. Helicobacter pylori promotes invasion and metastasis of gastric cancer by enhancing heparanase expression[J]. World J Gastroenterol,2018,24(40):4565-4577. doi: 10.3748/wjg.v24.i40.4565 -






 下载:
下载: