Studies on Cisplatin Resistance of miR-181a in Ovarian Cancer Cells
-
摘要:
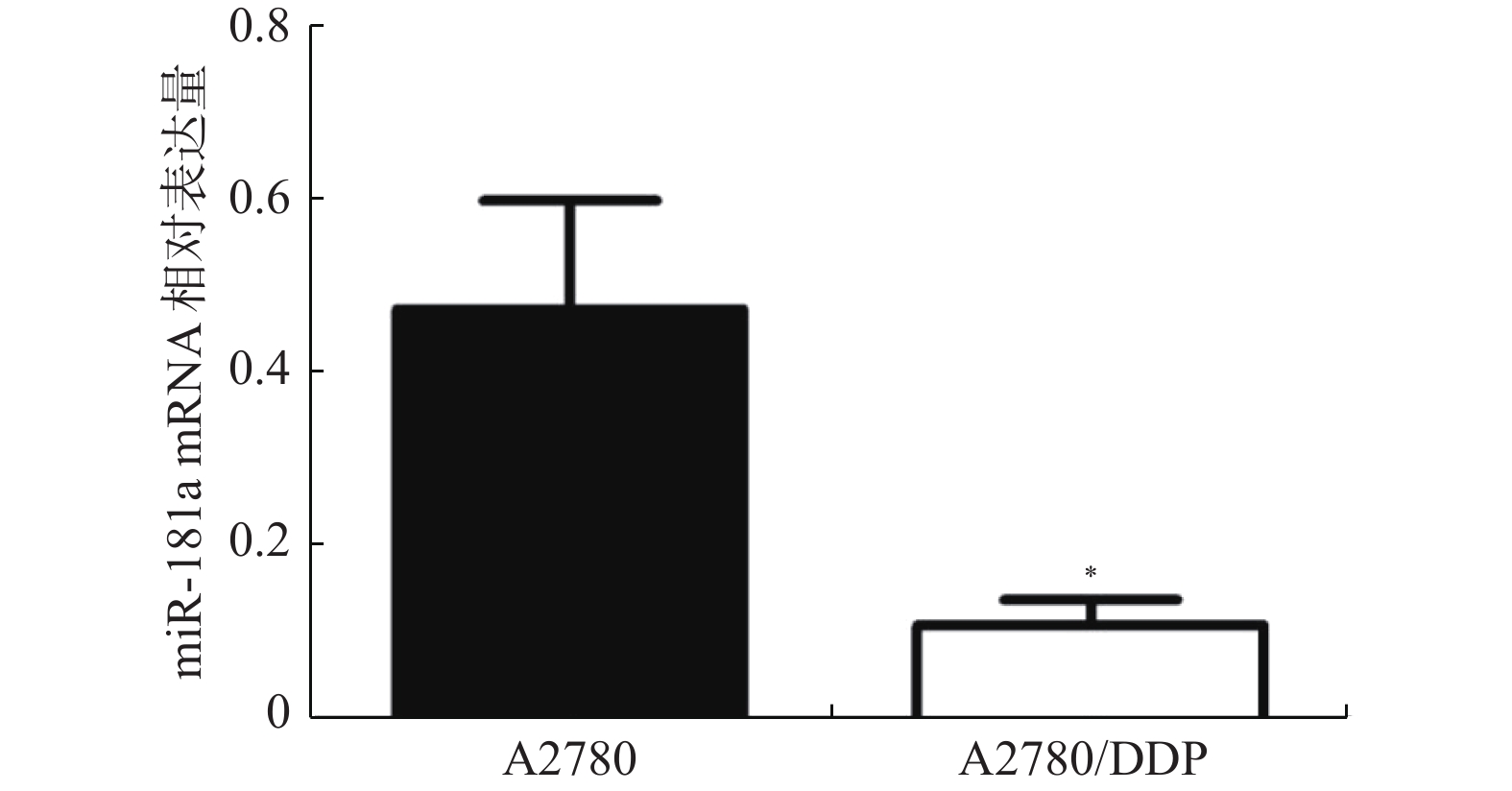
目的 探讨miR-181a在卵巢癌化疗抵抗方面的影响及其机制。 方法 qRT-PCR检测卵巢癌细胞A2780及A2780/DDP中miR-181a的表达;对A2780及A2780/DDP细胞分别进行miR-181a mimics和inhibitors的转染,并利用qRT-PCR技术验证;MTT法检测转染前后细胞对顺铂的敏感性,利用TargetScan、miRDB与miRwalk 3个MicroRNA靶基因数据库预测miR-181a下游靶点,并通过Western blot技术分析预测基因的表达。 结果 与A2780细胞相比,miR-181a在A2780/DDP细胞中的表达明显减弱(P < 0.05)。干扰miR-181a表达后,A2780细胞对顺铂的抵抗作用减弱(P < 0.05),而上调miR-181a表达,A2780/DDP细胞对顺铂的抵抗作用增强(P < 0.05)。通过TargetScan、miRDB、miRwalk三个数据库预测到PRKCD可作为miR-181a下游靶基因,下调miR-181a表达可使PRKCD蛋白表达明显增强(P < 0.05);反之,上调miR-181a表达能够显著抑制PRKCD蛋白表达(P < 0.05)。 结论 miR-181a卵巢癌细胞对顺铂的耐药性方面具有抑制作用。 Abstract:Objective To investigate the effect of miR-181a on chemotherapy resistance of ovarian cancer and its regulatory mechanism. Methods Real-time PCR was used to detect the expression of miR-181a in A2780 and cisplatin resistant cell lines (A2780/DDP), and siltation/overexpression changed the expression of miR-181a in A2780 and A2780/DDP cells. MTT method was used to determine the sensitivity of cells to cisplatin before and after the transfection. Three MicroRNA target gene databases, TargetScan, miRDB and miRwalk, were used to predict the downstream targets of miR-181a, and the changes in protein expression of target genes were analyzed by Western blot. Results Compared with A2780 cells, the expression of miR-181a in A2780/DDP cells was significantly decreased (P < 0.05). After the transfection of A2780 cells with miR-181a inhibitor (interfering with Mir-181A expression), the sensitivity of A2780 cells to cisplatin decreased (P < 0.05), and after the transfection of A2780/DDP cells with miR-181a mimic (overexpressing miR-181a), The sensitivity of cells to cisplatin was increased (P < 0.05). It was predicted that PRKCD could be used as the downstream target gene of miR-181a by TargetScan, miRDB and miRwalk databases, and down-regulation of miR-181a expression could significantly enhance PRKCD protein expression (P < 0.05). On the contrary, up-regulation of miR-181a significantly inhibited PRKCD protein expression (P < 0.05). Conclusion miR-181a may inhibit cisplatin resistance of ovarian cancer cell A2780 by regulating PRKCD. -
Key words:
- Ovarian cancer /
- PRKCD /
- miR-181a /
- Cisplatin resistance
-
表 1 A2780细胞在不同浓度顺铂作用下的MTT数值
Table 1. MTT results of A2780 cells treated with different concentrations of Cisplatin
组别 药物浓度(μmol/L) 0 10 20 30 40 50 阴性对照组 96.48 ± 1.58 73.42 ± 1.82 52.36 ± 1.32 19.87 ± 2.63 11.28 ± 2.36 6.68 ± 1.72 低表达组 97.23 ± 1.22 86.72 ± 1.76* 79.52 ± 1.68* 69.86 ± 1.92* 49.62 ± 1.56* 20.27 ± 1.82* t −3.007 −12.026 −130.674 −121.951 −83.009 −235.386 P 0.10 0.01 < 0.001 < 0.001 < 0.001 < 0.001 与阴性对照组比较,*P < 0.05。 表 2 A2780/DDP细胞在不同浓度顺铂作用下的MTT数值
Table 2. MTT results of A2780/DDP cells treated with different concentrations of Cisplatin
组别 药物浓度(μmol/L) 0 10 20 30 40 50 阴性对照组 97.42 ± 1.42 92.85 ± 1.72 85.64 ± 1.87 82.48 ± 2.24 76.95 ± 1.98 61.12 ± 2.32 低表达组 97.16 ± 1.82 88.63 ± 1.87 84.72 ± 1.87 76.12 ± 1.87 59.72 ± 2.52 40.58 ± 2.78 t 1.126 48.728 0.6025 29.773 55.265 77.34 P 0.377 < 0.001 0.579 < 0.001 < 0.001 < 0.001 -
[1] Amjadi M,Hallaj T,Hildebrandt N. A sensitive homogeneous enzyme assay for euchromatic histone-lysine-N-methyltransferase 2 (G9a) based on terbium-to-quantum dot time-resolved FRET[J]. Bioimpacts,2021,11(3):173-179. [2] Giordano M,Decio A,Battistini C,et al. L1CAM promotes ovarian cancer stemness and tumor initiation via FGFR1/SRC/STAT3 signaling[J]. J ExpClin Cancer Res,2021,40(1):319. [3] Zhang X,Qin T,Zhu Z,et al. Ivermectin augments the in vitro and in vivo efficacy of cisplatin in epithelial ovarian cancer by suppressing Akt/mTOR signaling[J]. Am J Med Sci,2020,359(2):123-129. [4] Hu Z,Cai M,Zhang Y,et al. miR-29c-3p inhibits autophagy and cisplatin resistance in ovarian cancer by regulating FOXP1/ATG14 pathway[J]. Cell Cycle (Georgetown,Tex. ),2020,19(2):193-206. [5] Forouhari S,Mahmoudi E,Safdarian E,et al. MicroRNA:A Potential Diagnosis for Male Infertility[J]. Mini Rev Med Chem,2021,21(10):1226-1236. doi: 10.2174/1389557520999201209213319 [6] Kim J,You S. Samul-tang ameliorates oocyte damage due to cyclophosphamide-induced chronic ovarian dysfunction in mice[J]. Sci Rep,2020,10(1):21925. doi: 10.1038/s41598-020-79013-7 [7] Jacob H,Stanisavljevic L,Storli K E,et al. Identification of a sixteen-microRNA signature as prognostic biomarker for stage II and III colon cancer[J]. Oncotarget,2017,8(50):87837-87847. doi: 10.18632/oncotarget.21237 [8] Huang F L,Liao E C,Li C L,et al. Pathogenesis of pediatric B-cell acute lymphoblastic leukemia: Molecular pathways and disease treatments[J]. Oncol Lett,2020,20(1):448-454. doi: 10.3892/ol.2020.11583 [9] Al-Hazmi A. Antioxidant activity of silymarin in microcystin-LR cardiac and pulmonary induced injuries on mice[J]. Pak J Biol Sci,2020,23(11):1369-1373. doi: 10.3923/pjbs.2020.1369.1373 [10] Lei B,Huang Y,Zhou Z,et al. Circular RNA hsa_circ_0076248 promotes oncogenesis of glioma by sponging miR-181a to modulate SIRT1 expression[J]. Cell Biochem,2019,120(4):6698-6708. doi: 10.1002/jcb.27966 [11] Abramson E,Hardman C,Shimizu A J,et al. Designed PKC-targeting bryostatin analogs modulate innate immunity and neuroinflammation[J]. Cell ChemBiol,2021,28(4):537-545.e4. [12] Ning Li,Long Bo,Zhang Wen,et al. Circular RNA profiling reveals circEXOC6B and circN4BP2L2 as novel prognostic biomarkers in epithelial ovarian cancer[J]. Int J Oncol,2018,53(6):2637-2646. [13] Giampaolino P,Della Corte L,Foreste V,et al. Unraveling a difficult diagnosis: The tricks for early recognition of ovarian cancer[J]. Minerva Med,2019,110(4):279-291. [14] Ni J,Zhang X,Li J,et al. Tumour-derived exosomal lncRNA-SOX2OT promotes bone metastasis of non-small cell lung cancer by targeting the miRNA-194-5p/RAC1 signalling axis in osteoclasts[J]. Cell Death Dis,2021,12(12):1131. [15] Ahn J H,Lee H S,Lee J S,et al. Nc886 is induced by TGF-β and suppresses the microRNA pathway in ovarian cancer[J]. Nat Commun,2018,9(1):1166. doi: 10.1038/s41467-018-07818-2 [16] 王皓,周宇. 微小RNA在肝胆胰疾病中作用的研究进展[J]. 实用肝脏病杂志,2011,14(2):155-157. doi: 10.3969/j.issn.1672-5069.2011.02.033 [17] De Paula Silva E,Marti L C,Andreghetto F M,et al. Extracellular vesicles cargo from head and neck cancer cell lines disrupt dendritic cells function and match plasma microRNAs[J]. Sci Rep,2021,11(1):18534. doi: 10.1038/s41598-021-97753-y [18] Gao Jianlong,Xia Silong. Reduced miR-519d-3p levels in the synovium and synovial fluid facilitate the progression of post-traumatic osteoarthritis by targeting VEGF[J]. Exp Ther Med,2021,22(6):1478. doi: 10.3892/etm.2021.10913 [19] 陈怡然. MicroRNA-181a靶向PRKCD调节子宫颈鳞癌化疗抵抗性的机制研究[D]. 上海: 复旦大学博士学位论文, 2013. [20] Ben-Mustapha I,Agrebi N,Barbouche M R. Novel insights into FAS defects underlying autoimmune lymphoproliferative syndrome revealed by studies in consanguineous patients[J]. J Leukoc Biol,2018,103(3):501-508. [21] Muscella A,Cossa L G,Vetrugno C,et al. Inhibition of ZL55 cell proliferation by ADP via PKC-dependent signalling pathway[J]. J Cell Physiol,2018,233(3):2526-2536. doi: 10.1002/jcp.26128 [22] Omarjee O,Picard C,Frachette C,et al. Monogenic lupus:Dissecting heterogeneity[J]. Autoimmun Rev,2019,18(10):102361. doi: 10.1016/j.autrev.2019.102361 [23] Munson M J,Mathai B J,Ng M Y W,et al. GAK and PRKCD are positive regulators of PRKN-independent mitophagy[J]. Nat Commun,2021,12(1):6101. doi: 10.1038/s41467-021-26331-7 [24] Chun K H,Cho S J,Lee J W,et al. Protein kinase C-δ interacts with and phosphorylates ARD1[J]. J Cell Physiol,2021,236(1):379-391. doi: 10.1002/jcp.29866 [25] Lothstein L,Soberman J,Parke D,et al. Pivarubicin is more effective than doxorubicin against triple-negative breast cancer in vivo[J]. Oncol Res,2020,28(5):451-465. doi: 10.3727/096504020X15898794315356 [26] Chen Y,Ke G,Han D,et al. MicroRNA-181a enhances the chemoresistance of human cervical squamous cell carcinoma to cisplatin by targeting PRKCD[J]. Exp Cell Res,2014,320(1):12-20. doi: 10.1016/j.yexcr.2013.10.014 -






 下载:
下载: