Effect of Surface Topography on Staphylococcus Epidermidis Biofilm Formation by Different 3D Printing Thickness of Biomaterials
-
摘要:
目的 探讨不同3D打印精度制作的生物材料表面形貌对表皮葡萄球菌生物膜形成的影响。 方法 以医用3D打印原材料光敏树脂MED610为材料,使用光固化成型技术分别按照16 μm、30 μm、100 μm层厚制作样本。测量样本表面粗糙轮廓的算术平均偏差 Ra、轮廓的最大高度Rz,静态角接触法检测样本疏水性。与表皮葡萄球菌标准株RP62A于振荡器上共培养,分别在2 h、6 h、12 h、24 h、30 h时取出材料表面,激光共聚焦显微镜测量单位视野细菌群落数量,扫描电镜观察材料表面生物膜形成情况。 结果 16 μm层厚制作生物材料表面Ra、Rz值较30 μm层厚与60 μm制作样本较小,不同层厚制作材料表面疏水性无明显差异(P > 0.05)。与表皮葡萄球菌共培养2 h、6 h时,16 μm组材料表面少量表皮葡萄球菌分散在材料表面,无细菌聚集现象出现,单位视野细菌群落数量明显低于30 μm组与100 μm组( P < 0.05)。培养12 h、24 h、30 h时,各组材料表面均可观察到生物膜形成,单位视野细菌群落数量无明显差异( P > 0.05)。 结论 在3D打印中以不同层厚制作出的材料表面粗糙度影响大,但对材料疏水性无明显影响。层厚越薄制作出的材料在感染早期不利于表皮葡萄球菌黏附。 Abstract:Objective To investigate the effect of surface topography on staphylococcus epidermidis biofilm formation by different 3D printing Thickness of biomaterials. Methods Medical 3D printing raw material, photosensitive resin MED610 was used as the material and samples were made according to 16 μm, 30 μm and 100 μm layer thicknesses using light-curing molding technology, respectively. Measure the sample surface roughness contour arithmetic mean deviation Ra, contour maximum height Rz, static angular contact method to detect sample hydrophobicity. The samples were co-cultured with Staphylococcus epidermidis standard strain RP62A on an oscillator, and the material surface was removed at 2 h, 6 h, 12 h, 24 h and 30 h, respectively. The number of bacterial communities per field of view was measured by laser confocal microscopy and the biofilm formation on the material surface was observed by scanning electron microscopy. Results The Ra and Rz values on the surface of the 16-μm layer thickness fabricated biomaterial were smaller than those of the 30-μm layer thickness and 60-μm fabricated samples, and there was no significant difference in the hydrophobicity of the surface of the fabricated materials with different layer thicknesses. When co-cultured with Staphylococcus epidermidis for 2 h and 6 h, a small amount of Staphylococcus epidermidis was scattered on the surface of the material in the 16 μm group without bacterial aggregation, and the number of bacterial communities per unit field of view was significantly lower than that in the 30 μm and 100 μm groups (P < 0.05). Biofilm formation could be observed on the material surface of each group at 12 h, 24 h and 30 h of incubation and there was no significant difference in the number of bacterial communities per unit field of view ( P > 0.05). Conclusions Different precision fabrication has a large effect on the surface roughness of 3D printing materials, but has no significant effect on hydrophobicity. The 16-μm layer thickness fabrication material is less favorable for Staphylococcus epidermidis adhesion in the early stage of infection than the 30-μm and 100-μm layer thickness fabrication materials. -
Key words:
- 3D Printing Technology /
- Biomaterials /
- Surface Topography /
- Staphylococcus Epidermidis /
- Biofilm
-
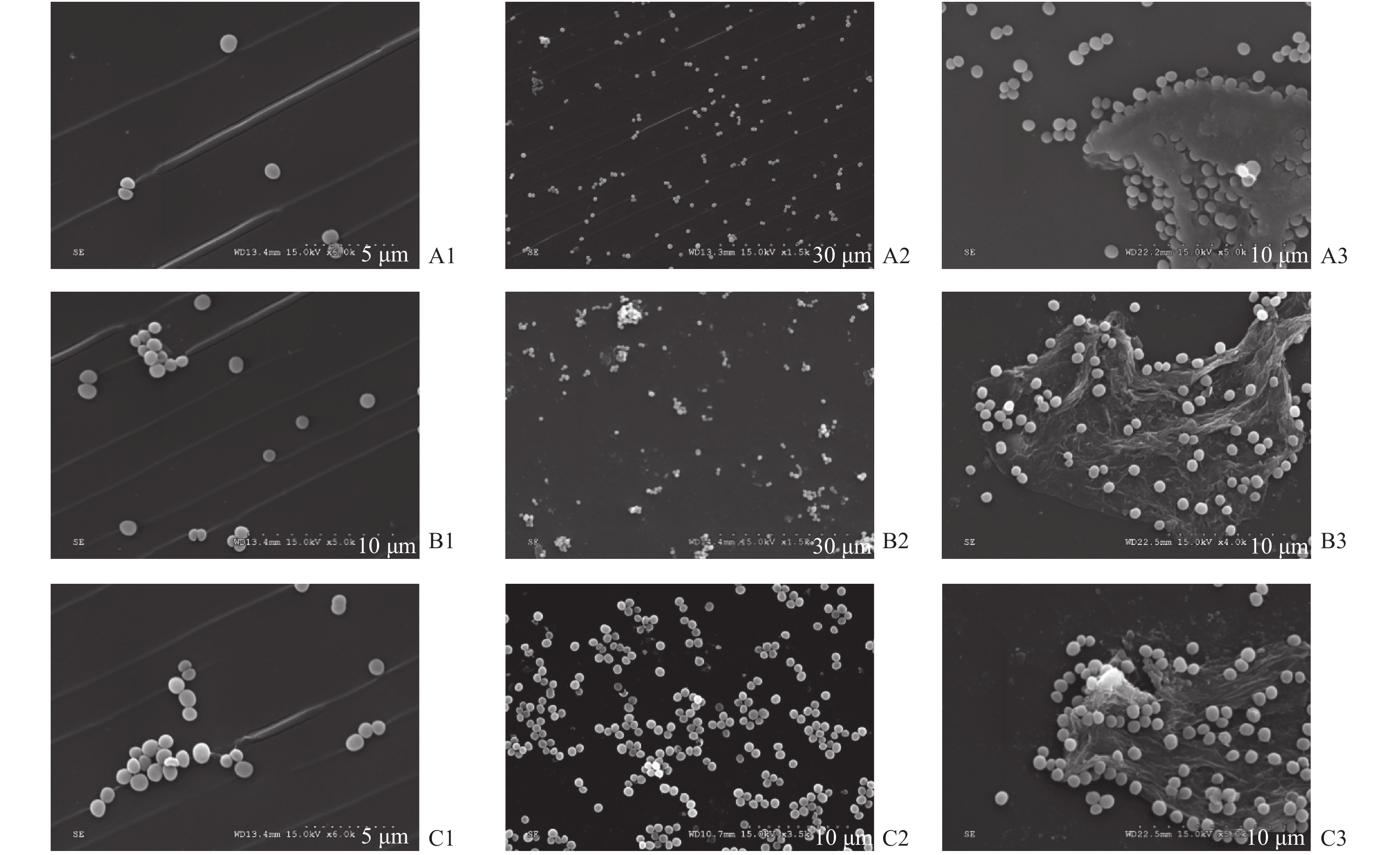
图 1 扫描电子显微镜(SEM)图像显示表皮葡萄球菌在不同层厚的材料表面不同时间形成的生物膜(10 µm)
A1:16 μm组2 h;A2:16 μm组6 h;A3:16 μm组30 h;B1:30 μm组2 h;B2:30 μm组6 h;B3:30 μm组30 h;C1:100 μm组2 h;C2:100 μm 组6 h;C3:100 μm 组30 h。
Figure 1. Scanning electron microscopy (SEM) images of biofilms formed by Staphylococcus epidermidis occure at different thicknesses and different times. Scale bar represented (10 µm)
表 1 不同层厚制作材料表面粗糙度(
$ \bar x \pm s $ )Table 1. Surface roughness of materials of different thickness (
$ \bar x \pm s $ )组别 Ra值 Rz值 16 μm 0.12 ± 0.03 0.51 ± 0.24 30 μm 0.19 ± 0.02 1.29 ± 0.36 100 μm 0.33 ± 0.03 2.32 ± 0.33 统计量 92.978 41.241 P值 < 0.001 < 0.001 表 2 不同层厚制作对材料疏水性影响(
$ \bar x \pm s $ )Table 2. Influence of different thickness on hydrophobicity of materials (
$ \bar x \pm s $ )组别 蒸馏水 甘油 16 μm 86.3 ± 1.6 76.8 ± 1.3 30 μm 84.5 ± 0.9 78.3 ± 1.6 100 μm 85.6 ± 1.3 78.9 ± 1.5 统计量 2.273 2.701 P值 0.146 0.108 表 3 同层厚制作材料表面单位视野表皮葡萄球菌群落数量(
$ \bar x \pm s $ )Table 3. Number of Staphylococcus epidermidis community per field of vision on the surface of the same thickness material (
$ \bar x \pm s $ )组别 2 h 6 h 12 h 24 h 30 h 16 μm 1.12 ± 0.72 4.71 ± 1.25 12.96 ± 1.95 18.62 ± 1.75 16.15 ± 2.03 30 μm 4.51 ± 1.04△ 8.95 ± 1.09△ 11.64 ± 1.56 19.39 ± 2.07 15.38 ± 1.85 100 μm 6.82 ± 1.25△ 10.86 ± 0.76△ 13.77 ± 2.05 20.44 ± 2.21 15.71 ± 2.15 统计量 38.965 44.891 1.676 1.016 0.180 P值 < 0.001 < 0.001 0.228 0.391 0.837 与16 μm组比较, △P < 0.05。 -
[1] Chen Y,Wang X Y,Huang Y C,et al. Study on the Structure of Candida Albicans-Staphylococcus Epidermidis Mixed Species Biofilm on Polyvinyl Chloride Biomaterial[J]. Cell Biochem Biophys,2015,73(2):461-468. doi: 10.1007/s12013-015-0672-y [2] Zhang J,Wehrle E,Rubert M,et al. 3D Bioprinting of Human Tissues:Biofabrication,Bioinks,and Bioreactors[J]. Int J Mol Sci,2021,22(8):3971. [3] 张慧,孟桐辉,刘琳,等. 3D生物打印材料在生物医学领域中的应用及研究进展[J]. 中华临床医师杂志(电子版),2019,13(02):157-160. doi: 10.3877/cma.j.issn.1674-0785.2019.02.015 [4] Liaw C Y,Guvendiren M. Current and emerging applications of 3D printing in medicine[J]. Biofabrication,2017,9(2):024102. doi: 10.1088/1758-5090/aa7279 [5] 车柯达,陈颖,雷玉洁. 3D打印技术在抗感染生物材料制作中的研究进展[J]. 重庆医学,2019,48(19):3359-3362. doi: 10.3969/j.issn.1671-8348.2019.19.029 [6] Niklason L E, Lawson J H. Bioengineered human blood vessels[J]. Science, 2020, 370: e6934. doi: 10.1155/202122083971. [7] Wang Z,Yang Y. Application of 3D Printing in Implantable Medical Devices[J]. Biomed Res Int,2021,2021:6653967. [8] Lei Y,Xu Y,Jing P,et al. The effects of TGF-beta1 on staphylococcus epidermidis biofilm formation in a tree shrew biomaterial-centered infection model[J]. Ann Transl Med,2021,9(1):57. doi: 10.21037/atm-20-4526 [9] Ensikat H J,Ditsche-Kuru P,Neinhuis C,et al. Superhydrophobicity in perfection:the outstanding properties of the lotus leaf[J]. Beilstein J Nanotechnol,2011,2:152-161. doi: 10.3762/bjnano.2.19 [10] 陈雅,叶联华,黄云超. 葡萄球菌生物膜形成影响因素的研究进展[J]. 中国医药导报,2017,14(36):37-34. [11] Engel A S,Kranz H T,Schneider M,et al. Biofilm formation on different dental restorative materials in the oral cavity[J]. BMC Oral Health,2020,20(1):162. doi: 10.1186/s12903-020-01147-x [12] Montelongo-Jauregui D,Srinivasan A,Ramasubramanian A K,et al. An in vitro model for oral mixed biofilms of Candida albicans and Streptococcus gordonii in synthetic saliva[J]. Frontiers in microbiology,2016,7:686. [13] De-La-Pinta I,Cobos M,Ibarretxe J,et al. Effect of biomaterials hydrophobicity and roughness on biofilm development[J]. J Mater Sci Mater Med,2019,30(7):77. doi: 10.1007/s10856-019-6281-3 [14] Kligman S,Ren Z,Chung C H,et al. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation[J]. J Clin Med,2021,10(8):1641. [15] Linklater D P,Baulin V A,Juodkazis S,et al. Mechano-bactericidal actions of nanostructured surfaces[J]. Nat Rev Microbiol,2021,19(1):8-22. doi: 10.1038/s41579-020-0414-z [16] Tripathy A,Sen P,Su B,et al. Natural and bioinspired nanostructured bactericidal surfaces[J]. Adv Colloid Interface Sci,2017,248:85-104. doi: 10.1016/j.cis.2017.07.030 [17] Ismail H S,Ali A I,Abo El-Ella M A,et al. Effect of different polishing techniques on surface roughness and bacterial adhesion of three glass ionomer-based restorative materials:In vitro study[J]. J Clin Exp Dent,2020,12(7):e620-e625. [18] Singh T,Hook A L,Luckett J,et al. Discovery of hemocompatible bacterial biofilm-resistant copolymers[J]. Biomaterials,2020,260:120312. doi: 10.1016/j.biomaterials.2020.120312 [19] Mccarthy R R,Ullah M W,Pei E,et al. Antimicrobial Inks:The Anti-Infective Applications of Bioprinted Bacterial Polysaccharides[J]. Trends Biotechnol,2019,37(11):1155-1159. doi: 10.1016/j.tibtech.2019.05.004 [20] Liu J,Yao X,Ye J,et al. A printing-spray-transfer process for attaching biocompatible and antibacterial coatings to the surfaces of patient-specific silicone stents[J]. Biomed Mater,2020,15(5):1748. doi: 10.1088/1748-605X/ab99d6 [21] Zhang Y,Zhai D,Xu M,et al. 3D-printed bioceramic scaffolds with antibacterial and osteogenic activity[J]. Biofabrication,2017,9(2):1758-5090. doi: 10.1088/1758-5090/aa6ed6 -






 下载:
下载: