Value of Multiple Scoring Systems in Evaluating the Prognosis of Liver Cirrhosis Complicated with Esophageal and Gastric Varices Bleeding
-
摘要:
目的 比较AIMS65、GBS、MGBS、EGBS、CRS、CANUKA、Child-Turcotte-Pugh(CTP)、MELD、MELD-Na评分系统在肝硬化合并食管胃底静脉曲张破裂出血患者(esophagealgastricvariceal bleeding,EGVB)预后评估中的价值,探讨影响肝硬化合并EGVB患者预后不良的影响因素。 方法 对确诊为肝硬化合并EGVB的患者169例,根据患者是否发生院内再出血或死亡将患者分为预后良好组和预后不良组,计算出每名患者入院时的各模型评分,比较2组患者的临床特点。 结果 预后不良组35例,预后良好组134例,预后不良组的评分均高于预后良好组,AIMS65评分在预测患者是否预后不良时表现最优,且差异具有统计学意义(P < 0.05)。预后良好组患者HB、ALB较预后不良组高,PT、INR较预后不良组低,差异具有统计学意义(P < 0.05),经多因素Logistic回归分析提示ALB可能是肝硬化合并EGVB患者预后不良的独立保护因素。 结论 AIMS65是肝硬化合并食管胃底静脉曲张出血患者预后评估的最佳评分系统;ALB可能是肝硬化合并食管胃底静脉曲张出血患者预后不良的独立保护因素。 -
关键词:
- 肝硬化 /
- 食管胃底静脉曲张出血 /
- 评分系统
Abstract:Objective To compare the values of AIMS65, GBS, MGBS, EGBS, CRS, CANUKA, Child-Turcotte-Pugh (CTP), MELD, and MELD-Na scoring systems in the evaluation of the prognosis of patients with liver cirrhosis and EGVB, and explore the factors affecting the poor prognosis of patients with liver cirrhosis and EGVB. Methods A total of 169 patients diagnosed with liver cirrhosis and EGVB were divided into a good prognosis group and a poor prognosis group according to whether the patients had rebleeding or death in the hospital. The scores of each model at the time of admission of each patient were calculated, and the clinical characteristics of the two groups of patients were compared. Results There were 35 cases in the poor prognosis group and 134 cases in the good prognosis group. The scores of the poor prognosis group were higher than those of the good prognosis group. The AIMS65 score was the best in predicting whether the patient had a poor prognosis, and the difference was statistically significant (P < 0.05). The HB and ALB in the good prognosis group were higher than those in the poor prognosis group, and the PT and INR were lower than those in the poor prognosis group. The difference was statistically significant (P < 0.05). Multivariate logistic regression analysis suggested that ALB may be an independent protection factor for poor prognosis in patients with liver cirrhosis and EDVB. Conclusion AIMS65 is the best scoring system for prognostic evaluation of patients with liver cirrhosis and esophageal varices bleeding; ALB may be an independent protective factor for the poor prognosis of patients with liver cirrhosis and esophageal and gastric varices bleeding. -
Key words:
- Hepatic cirrhosis /
- Esophageal gastric variceal bleeding /
- Scoring system
-
表 1 预后不良患者与预后良好患者的一般情况对比[(
$\bar x \pm s $ )/M(P25,P75)/n(%)]Table 1. Comparison of general conditions of patients with poor prognosis and patients with good prognosis [(
$\bar x \pm s $ )/M(P25,P75)/n(%)]指标 预后不良组(n = 35) 预后良好组(n = 134) t/z P 年龄(岁) 52.00(47.00,63.00) 54.00(46.75,65.00) −0.116 0.907 性别(男/女) 21/14 87/47 0.292 0.589 心率(次/min) 91.26 ± 18.31 86.69 ± 15.67 1.480 0.141 收缩压(mmHg) 107.00(98.00,124.00) 111.00(101.00,124.25) −0.825 0.410 合并糖尿病 6(17.14) 18(13.43) 0.083 0.773 合并肿瘤 11(31.43) 26(19.40) 2.347 0.126 表 2 不良预后组与预后良好组各项指标单因素分析[(
$\bar x \pm s $ )/M(P25,P75)]Table 2. Single factor analysis of various indicators in poor prognosis group and good prognosis group [(
$\bar x \pm s $ )/M(P25,P75)]指标 预后不良组(n = 35) 预后良好组(n = 134) t/z P 血红蛋白(g/L) 74.57 ± 19.87 86.31 ± 25.40 −2.536 0.012* 白细胞(×109/L) 5.68(3.61,9.11) 4.78(3.18,7.05) −1.389 0.165 血小板(×109/L) 81.00(57.00,113.00) 80.00(59.75,119.50) −0.107 0.915 白蛋白(g/L) 25.28 ± 5.69 30.05 ± 5.85 −4.314 < 0.001* 总胆红素(μmol/L) 28.40(18.30,49.80) 24.95(16.58,35.43) −1.629 0.103 尿素(mmol/L) 8.58(5.30,11.82) 6.71(4.87,10.06) −1.847 0.065 肌酐(μmol/L) 68.00(56.00,88.00) 65.50(53.75,79.50) −0.990 0.322 PT(s) 18.10(15.80,21.70) 16.30(15.08,17.93) −3.135 0.002* INR 1.52(1.35,1.92) 1.33(1.21,1.49) −3.453 0.001* Na+(mmol/L) 137.20(135.50,139.20) 138.15(136.38,140.55) −1.618 0.106 ALT(U/L) 36.00(26.00,49.00) 36.50(27.00,51.00) −0.008 0.994 AST(U/L) 45.00(27.00,74.00) 43.00(27.75,65.00) −0.634 0.526 *P < 0.05。 表 3 预后不良影响因素的二分类Logistic回归分析
Table 3. Binary logistic regression analysis of factors influencing poor prognosis
指标 B SE Wald df P OR OR的95%CI 下限 上限 Hb −0.004 0.010 0.135 1 0.713 0.996 0.978 1.015 ALB −0.116 0.047 6.221 1 0.013 0.890 0.813 .975 PT −0.721 0.662 1.187 1 0.276 0.486 0.133 1.779 INR×100 0.070 0.059 1.393 1 0.238 1.072 0.955 1.203 表 4 预后不良组和预后良好组中九种评分系统的分值与对比[M(P25,P75)]
Table 4. Comparison of scores of nine scoring systems in the poor prognosis group and the good prognosis group [M(P25,P75)]
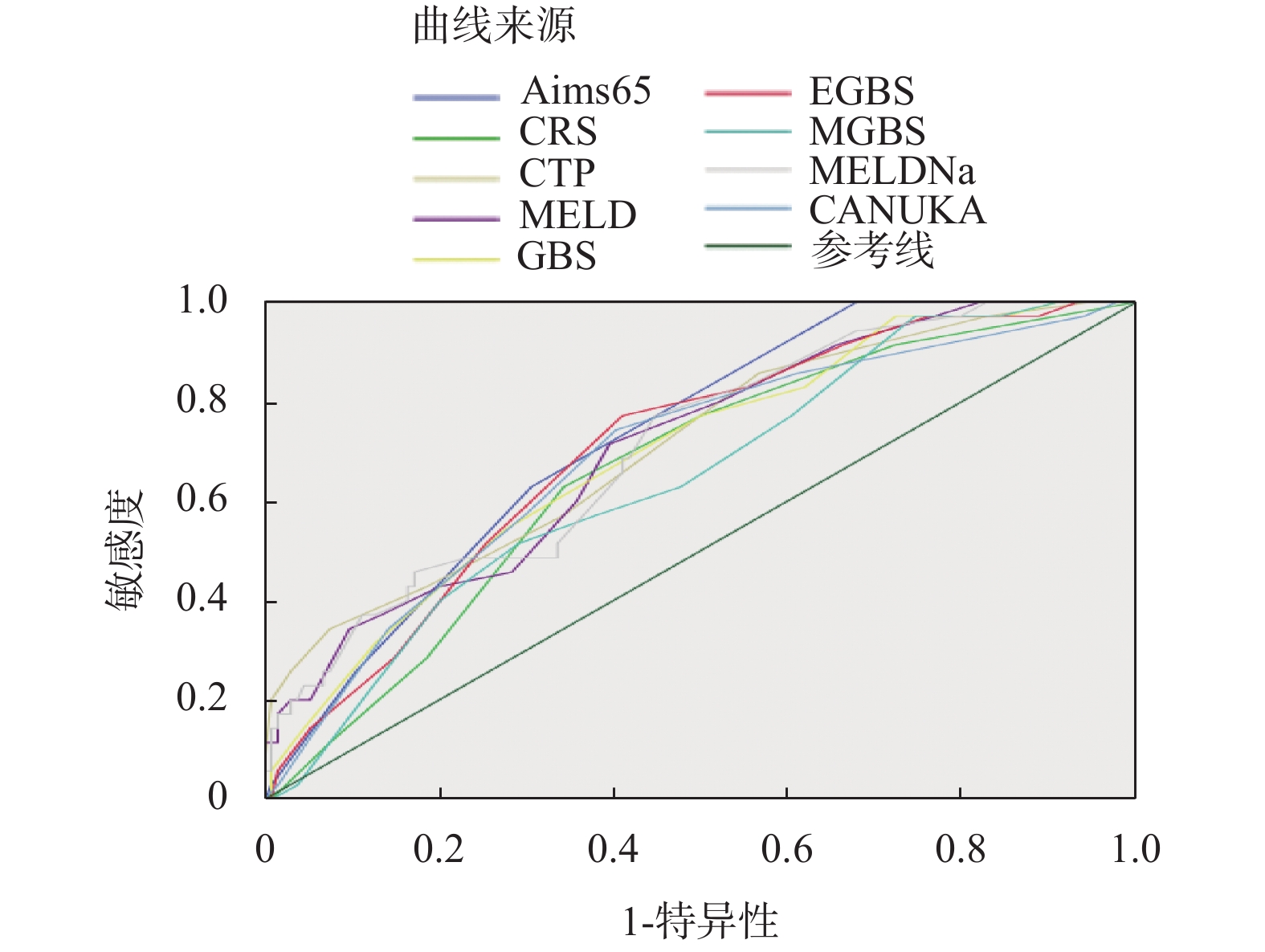
评分系统 预后不良组(n = 35) 预后良好组(n = 134) z P AIMS65 2.00(1.00,3.00) 1.00(0.00,2.00) −4.33 < 0.001* GBS 13.00(11.00,15.00) 10.50(8.00,13.00) −3.56 < 0.001* MGBS 10.00(7.00,11.00) 7.00(5.00,10.00) −2.67 0.008* EGBS 14.00(12.00,16.00) 11.00(9.00,14.00) −3.73 < 0.001* CRS 3.00(2.00,4.00) 1.50(0.00,3.00) −2.98 0.003* CANUKA 10.00(8.00,11.00) 8.00(7.00,10.00) −3.51 < 0.001* CTP 9.00(8.00,12.00) 8.00(7.00,9.00) −3.88 < 0.001* MELD 12.00(10.00,16.00) 10.00(8.00,13.00) −3.82 < 0.001* MELD-Na 12.07(11.00,17.75) 10.00(8.00,13.80) −3.83 < 0.001* *P < 0.05。 表 5 各评分系统对肝硬化EGVB患者预后不良预测价值的对比
Table 5. Comparison of the predictive value of each scoring system for the poor prognosis of patients with liver cirrhosis and EGVB
评分 AUROC 95%CI AUROC差值 95%CI P AIMS65 0.728 0.654~0.793 参考 CTP 0.710 0.635~0.777 0.0181 −0.0634~0.0996 0.6630 MELD-Na 0.710 0.635~0.777 0.0179 −0.0683~0.104 0.6840 MELD 0.709 0.634~0.776 0.0190 −0.0701~0.108 0.6762 EGBS 0.704 0.629~0.772 0.0237 −0.0848~0.132 0.6689 GBS 0.695 0.619~0.763 0.0332 −0.0860~0.152 0.5854 CANUKA 0.691 0.615~0.760 0.0371 −0.0764~0.151 0.5219 CRS 0.661 0.584~0.732 0.0674 −0.0500~0.185 0.2604 MGBS 0.646 0.569~0.718 0.0820 −0.0393~0.203 0.1853 表 6 各评分系统对肝硬化EGVB患者预后不良的预测价值及最佳阈值
Table 6. The predictive value and optimal threshold of each scoring system for poor prognosis of patients with liver cirrhosis and EDVB
评分 AUROC Youdenindex Cut-off SE 95%CI Sp 95%CI AIMS65 0.728 0.3226 > 1 62.86 44.9~78.5 69.40 60.9~77.1 CTP 0.710 0.2900 > 7 85.71 69.7~95.2 43.28 34.8~52.1 MELD-Na 0.710 0.3237 > 10 77.14 59.9~89.6 55.22 46.4~63.8 MELD 0.709 0.3188 > 10 71.43 53.7~85.4 60.45 51.6~68.8 EGBS 0.704 0.3610 > 11 77.14 59.9~89.6 58.96 50.1~67.4 GBS 0.695 0.2714 > 10 77.14 59.9~89.6 50.00 41.2~58.8 CANUKA 0.691 0.3399 > 8 74.29 56.7~87.5 59.70 50.9~68.1 CRS 0.661 0.2853 > 2 62.86 44.9~78.5 65.67 57.0~73.7 MGBS 0.646 0.2252 > 5 97.14 85.1~99.9 25.37 18.3~33.6 -
[1] Mouelhi L,Ayadi H,Zaimi Y,et al. Predictive scores of early mortality from variceal gastrointestinal bleeding in cirrhotic patients[J]. Tunis Med,2016,94(11):670-674. [2] 肝硬化门静脉高压症食管、胃底静脉曲张破裂出血诊治专家共识(2019版)[J]. 中华外科杂志, 2019, 57(12): 885-892. [3] Jeon H J,Moon H S,Kwon I S,et al. Which scoring system should be used for non-variceal upper gastrointestinal bleeding? Old or new?[J]. Gastroenterol Hepatol,2021,36(10):2819-2827. doi: 10.1111/jgh.15555 [4] Stanley AJ,Laine L,Dalton HR,et al. International Gastrointestinal Bleeding Consortium. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding:international multicentre prospective study[J]. BMJ,2017,4(7):356-359. [5] Kawaguchi K,Isomoto H. Validation of AIMS65 to predict outcomes in acute variceal bleeding:Which risk scoring system outperforms in real practice?[J]. Dig Endosc,2020,32(5):739-741. doi: 10.1111/den.13657 [6] Biselli M,Gramenzi A,Lenzi B,et al. Development and Validation of a Scoring System That Includes Corrected QT Interval for Risk Analysis of Patients With Cirrhosis and Gastrointestinal Bleeding[J]. Clin Gastroenterol Hepatol,2019,17(7):1388-1397. doi: 10.1016/j.cgh.2018.12.006 [7] Tantai X X,Liu N,Yang L B,et al. Prognostic value of risk scoring systems for cirrhotic patients with variceal bleeding[J]. World J Gastroenterol,2019,25(45):6668-6680. doi: 10.3748/wjg.v25.i45.6668 [8] Robertson M,Ng J,Abu Shawish W,et al. Risk stratification in acute variceal bleeding:Comparison of the AIMS65 score to established upper gastrointestinal bleeding and liver disease severity risk stratification scoring systems in predicting mortality and rebleeding[J]. Dig Endosc,2020,32(5):761-768. doi: 10.1111/den.13577 [9] Motola-Kuba M,Escobedo-Arzate A,Tellez-Avila F,et al. Validation of prognostic scores for clinical outcomes in cirrhotic patients with acute variceal bleeding[J]. Ann Hepatol,2016,15(6):895-901. [10] Budimir I,Gradišer M,Nikolić M,et al. Glasgow Blatchford,pre-endoscopic Rockall and AIMS65 scores show no difference in predicting rebleeding rate and mortality in variceal bleeding[J]. Scand J Gastroenterol,2016,51(11):1375-1379. doi: 10.1080/00365521.2016.1200138 [11] 中华医学会肝病学分会. 肝硬化诊治指南[J]. 现代医药卫生,2020,36(2):1-19. [12] Saltzman John R,Tabak Ying P,Hyett Brian H,et al. A simple risk score accurately predicts in-hospital mortality,length of stay,and cost in acute upper GI bleeding[J]. Gastrointest Endosc,2011,74(6):1215-1224. doi: 10.1016/j.gie.2011.06.024 [13] Blatchford O,Murray W R,Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage[J]. Lancet,2000,356(9238):1318-1321. doi: 10.1016/S0140-6736(00)02816-6 [14] Cheng D W,Lu Y W,Teller T,et al. A modified Glasgow Blatchford Score improves risk stratification in upper gastrointestinal bleed:a prospective comparison of scoring systems[J]. Aliment Pharmacol Ther,2012,36(8):782-789. doi: 10.1111/apt.12029 [15] Laursen S B,Hansen J M,Schaffalitzky de Muckadell O B. The Glasgow Blatchford score is the most accurate assessment of patients with upper gastrointestinal hemorrhage[J]. Clin Gastroenterol Hepatol,2012,10(10):1130-1135. doi: 10.1016/j.cgh.2012.06.022 [16] Rockall T A,Logan R F,Devlin H B,et al. Risk assessment after acute upper gastrointestinal haemorrhage[J]. Gut,1996,38(3):316-321. doi: 10.1136/gut.38.3.316 [17] Oakland K,Kahan B C,Guizzetti L,et al. Development,validation,and comparative assessment of an international scoring system to determine risk of upper gastrointestinal bleeding[J]. Clin Gastroenterol Hepatol,2019,17(6):1121-1129. doi: 10.1016/j.cgh.2018.09.039 [18] Huo T I,Wang Y W,Yang Y Y,et al. Model for end-stage liver disease score to serum sodium ratio index as a prognostic predictor and its correlation with portal pressure in patients with liver cirrhosis[J]. Liver Int,2007,27(4):498-506. doi: 10.1111/j.1478-3231.2007.01445.x [19] Morales-Arráez D,Ventura-Cots M,Altamirano J,et al. The MELD Score Is Superior to the Maddrey Discriminant Function Score to Predict Short-Term Mortality in Alcohol-Associated Hepatitis:A Global Study[J]. Gastroenterol,2022,117(2):301-310. [20] Ruf A E,Kremers W K,Chavez L L,et al. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone[J]. Liver Transpl,2005,11(3):336-343. doi: 10.1002/lt.20329 [21] 程家宁. 内镜联合药物诊治肝硬化食管-胃底静脉曲张破裂出血的疗效及再出血影响因素[J]. 临床普外科电子杂志,2021,9(4):26-30. doi: 10.3969/j.issn.2095-5308.2021.04.006 [22] 海静,罗和生. 几种非侵入性肝纤维化评分系统在肝硬化并食管胃底静脉曲张破裂出血组与非出血组间的比较[J]. 胃肠病学和肝病学杂志,2021,30(7):811-814+821. [23] 苏争艳,孙超,蒋肸慧,等. 三种评分系统在肝硬化食管胃底静脉曲张破裂出血患者风险评估中的应用[J]. 中华消化内镜杂志,2020,37(2):105-110. [24] Mihas A A,Sanyal A J. Recurrent variceal bleeding despite endoscopic and medical therapy[J]. Gastroenterology,2004,127(2):621-629. doi: 10.1053/j.gastro.2004.05.060 [25] 江秋维,黄理,姚朝光. 乙型肝炎肝硬化并发食管胃底静脉曲张破裂出血患者临床特征及其危险因素分析[J]. 实用肝脏病杂志,2021,24(4):532-535. doi: 10.3969/j.issn.1672-5069.2021.04.020 [26] 余维微,蔡宗宗,曾耀明,等. 3种评分系统对肝硬化胃底食管静脉曲张出血患者预后的预测价值[J]. 重庆医学,2020,49(10):1623-1626+1630. [27] 朱思奇,赵祥安,王甦. 肝硬化伴食管胃底静脉曲张出血的影响因素及3种模型对再出血的预测价值[J]. 实用临床医药杂志,2021,25(16):49-53. [28] Qi X,Su C,Ren W,et al. Association between portal vein thrombosis and risk of bleeding in liver cirrhosis:A systematic review of the literature[J]. Clin Res Hepatol Gastroenterol,2015,39(6):683-691. doi: 10.1016/j.clinre.2015.02.012 [29] 王亮. 肝硬化门静脉高压症术后门静脉血栓形成危险因素及干预措施分析[J]. 现代中西医结合杂志,2017,26(6):626-628. doi: 10.3969/j.issn.1008-8849.2017.06.020 [30] Krige J E,Kotze U K,Distiller G,et al. Predictive factors for rebleeding and death in alcoholic cirrhotic patients with acute variceal bleeding:a multivariate analysis[J]. World J Surg,2009,33(10):2127-2135. doi: 10.1007/s00268-009-0172-6 [31] Iino C,Shimoyama T,Igarashi T,et al. Usefulness of the Glasgow-Blatchford score to predict 1-week mortality in patients with esophageal variceal bleeding[J]. Eur J Gastroenterol Hepatol,2017,29(5):547-551. doi: 10.1097/MEG.0000000000000844 -






 下载:
下载: