Potential Mechanism of Thioridazine in Anti-cervical Cancer
-
摘要:
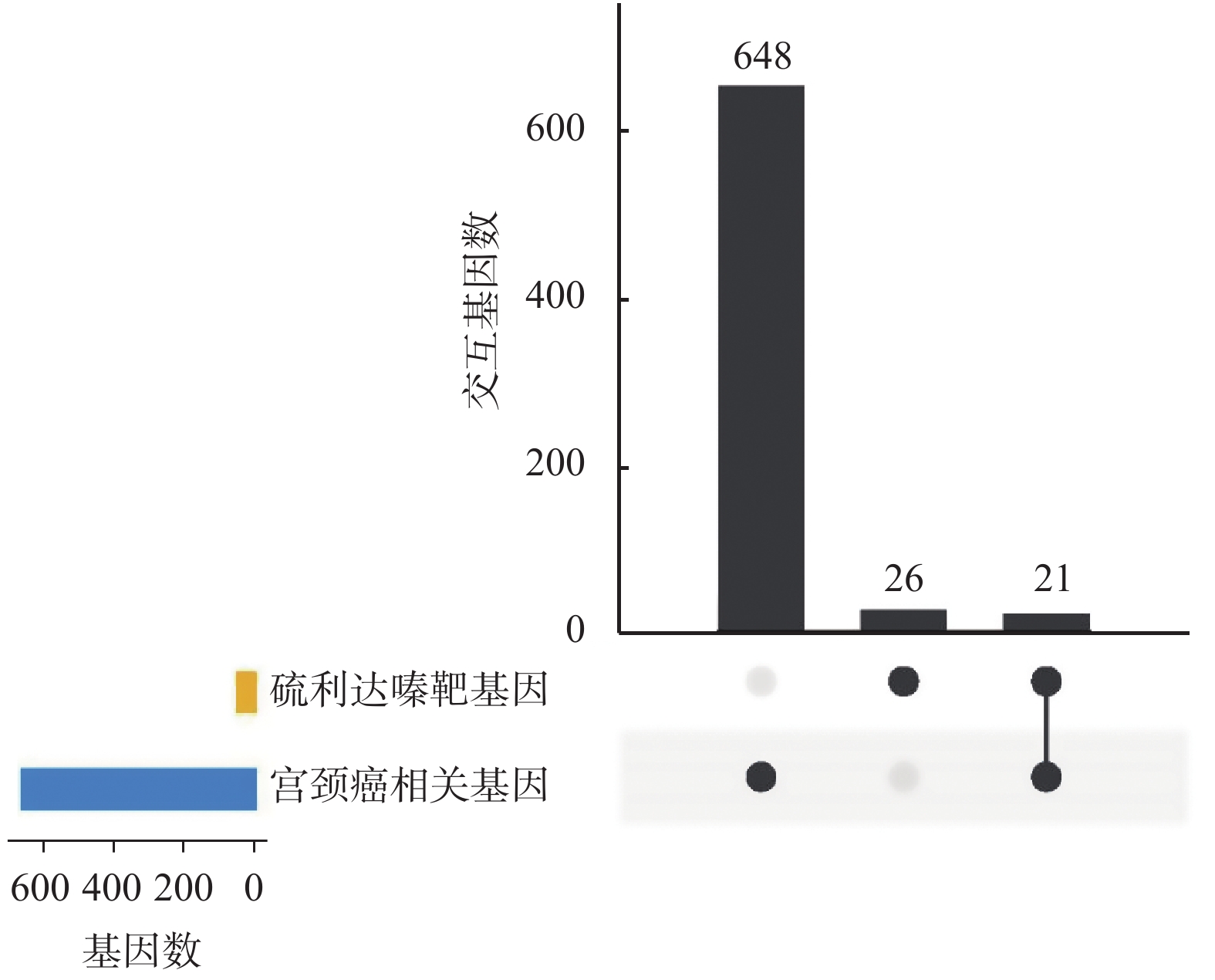
目的 运用生物信息学分析, 探讨硫利达嗪抗宫颈癌的潜在作用机制。 方法 通过PharmMapper工具预测能够与硫利达嗪相互作用的靶基因,然后使用STRING在线工具对靶基因进行通路和组织表达富集分析。使用GeneCards和DisGeNET数据库筛选宫颈癌相关基因,与硫利达嗪的靶基因取交集,得到硫利达嗪可能作用于宫颈癌的交互基因。通过STRING构建蛋白互作(PPI)网络,推测核心靶点,评估其重要性。使用clusterProfiler软件进行GO和KEGG通路分析。 结果 通过PharmMapper预测得到47个靶基因,富集到肿瘤相关通路和宫颈癌细胞。与669个宫颈癌基因取交集,获得硫利达嗪和宫颈癌交互基因21个,其中10个关键节点基因为EGFR、PPARG、AR、NOS3、ALB、ESR1、MAPK1、MAPK14、ANXA5和MAPK8,且在宫颈癌PPI网络中处于重要位置。交互基因涉及到的生物进程主要有负离子转运的正调控、丝氨酸肽基磷酸化、类固醇代谢过程、血液凝固、细胞对化学应激的反应等。富集的KEGG通路包括调控癌症通路、松弛素信号通路、内分泌耐药、蛋白聚糖与肿瘤、细胞衰老、GnRH信号通路和VEGF信号通路等。 结论 硫利达嗪具有潜在的抗肿瘤作用,可能通过多靶点和多信号通路的方式在宫颈癌的治疗上发挥作用。 Abstract:Objective To explore the potential mechanism of thioridazine in the treatment of cervical cancer by bioinformatics analysis. Methods The target genes that can interact with thioridazine was predicted by PharmMapper, and then conducted pathway and tissue expression enrichment analysis by STRING online tool. The cervical cancer related genes were screened through GeneCards and DisGeNET databases, and cross the target genes of thioridazine to obtain the interaction genes that thioridazine may act on cervical cancer. The protein-protein interaction(PPI) network was constructed using STRING, and the core targets were speculated and evaluated their importance.The GO and KEGG enrichment analysis were conducted using clusterProfiler package. Results A total of 47 target genes were predicted by PharmMapper, which were enriched in tumor-related pathways and cervical cancer cells. Intersection with 669 cervical cancer genes, 21 common genes were obtained, of which 10 key genes were EGFR, PPARG, AR, NOS3, ALB, ESR1, MAPK1, MAPK14, ANXA5 and MAPK8. These key genes were important in the cervical cancer PPI network. The biological processes involved in interactive genes mainly include positive regulation of anion transport, peptidyl-serine phosphorylation, steroid metabolic process, blood coagulation, and cellular response to chemical stress. Enriched KEGG pathways include pathways in cancer, relaxin signaling pathway, endocrine resistance, proteoglycans in cancer, cellular senescence, GnRH signaling pathway, and VEGF signaling pathway. Conclusion Thioridazine has potential anti-tumor effects and may play a role in the treatment of cervical cancer through multiple targets and multiple signaling pathways. -
Key words:
- Thioridazine /
- Cervical cancer /
- Target /
- Action mechanism
-
表 1 排名前10的靶基因通路分析结果
Table 1. Top10 pathways results of target genes
ID号 名称 基因数 FDR hsa04926 松弛素信号通路 9 1.50E-08 hsa05200 肿瘤通路 13 4.88E-08 hsa01522 内分泌耐药 7 6.70E-07 hsa05205 蛋白聚糖与肿瘤 8 3.01E-06 hsa05166 人类T细胞白血病
病毒1感染8 4.18E-06 hsa04218 细胞衰老 7 6.70E-06 hsa04912 促性腺激素释放
激素信号通路6 6.70E-06 hsa04914 孕酮介导的卵
母细胞成熟6 7.36E-06 hsa05161 乙型肝炎 7 7.36E-06 hsa04933 糖尿病并发症中
的AGE-RAGE信号通路6 7.85E-06 表 2 排名前10的靶基因疾病富集分析结果
Table 2. Top10 disease enrichment results of target genes
ID号 名称 基因数 FDR DOID:162 肿瘤 12 0.0022 DOID:14566 细胞增殖疾病 13 0.0022 DOID:4 疾病 31 0.0022 DOID:65 结缔组织疾病 10 0.0064 DOID:7 解剖实体疾病 25 0.0064 DOID:0050636 家族性内脏
淀粉样变性3 0.0183 DOID:28 内分泌系统疾病 7 0.0183 DOID:0080001 骨疾病 8 0.0184 DOID:11801 蛋白质-能量营
养不良2 0.0184 DOID:0060075 雌激素受体
阳性乳腺癌2 0.0244 表 3 排名前10的靶基因组织表达富集分析
Table 3. Top10 tissue expression enrichment results of target genes
ID号 名称 基因数 FDR BTO:0000180 宫颈癌细胞 10 2.03E-06 BTO:0000174 胚胎结构 22 2.03E-06 BTO:0000759 肝 20 2.71E-06 BTO:0000132 血小板 10 4.54E-06 BTO:0001078 胎盘 16 4.54E-06 BTO:0001546 慢性淋巴细胞
白血病细胞8 8.77E-06 BTO:0001489 全身 46 9.93E-06 BTO:0000345 消化腺 22 1.02E-05 BTO:0001491 脏器 30 1.07E-05 BTO:0000522 腺体 34 1.77E-05 表 4 关键靶基因在宫颈癌PPI网络中的排名
Table 4. Ranking of key target genes in the cervical cancer PPI network
靶基因 Degree值 排名 EGFR 329 6 ALB 271 13 ESR1 255 20 ANXA5 200 32 PPARG 182 37 AR 159 53 MAPK1 143 72 MAPK14 141 77 MAPK8 138 79 NOS3 105 127 合计 525 -
[1] Sung H,Ferlay J,Siegel R L,et al. Global cancer statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2021,71(3):209-249. doi: 10.3322/caac.21660 [2] de Foucher T,Bendifallah S,Ouldamer L,et al. Patterns of recurrence and prognosis in locally advanced FIGO stage IB2 to IIB cervical cancer:Retrospective multicentre study from the FRANCOGYN group[J]. Eur J Surg Oncol,2019,45(4):659-665. doi: 10.1016/j.ejso.2018.11.014 [3] Fenton M,Rathbone J,Reilly J,et al. Thioridazine for schizophrenia[J]. Cochrane Database Syst Rev,2007,2007(3):CD001944. [4] Duarte D,Cardoso A,Vale N. Synergistic growth inhibition of HT-29 colon and MCF-7 breast cancer cells with simultaneous and sequential combinations of antineoplastics and CNS drugs[J]. Int J Mol Sci,2021,22(14):7408. doi: 10.3390/ijms22147408 [5] Tegowski M,Fan C,Baldwin A S. Selective effects of thioridazine on self-renewal of basal-like breast cancer cells[J]. Sci Rep,2019,9(1):18695. doi: 10.1038/s41598-019-55145-3 [6] Mu J,Xu H,Yang Y,et al. Thioridazine,an antipsychotic drug,elicits potent antitumor effects in gastric cancer[J]. Oncol Rep,2014,31(5):2107-2114. doi: 10.3892/or.2014.3068 [7] Lu M,Li J,Luo Z,et al. Roles of dopamine receptors and their antagonist thioridazine in hepatoma metastasis[J]. Onco Targets Ther,2015,8(default):1543-1552. [8] Cheng H W,Liang Y H,Kuo Y L,et al. Identification of thioridazine,an antipsychotic drug,as an antiglioblastoma and anticancer stem cell agent using public gene expression data[J]. Cell Death Dis,2015,6(5):e1753. doi: 10.1038/cddis.2015.77 [9] Hercbergs A. Thioridazine:a radiation enhancer in advanced cervical cancer?[J]. Lancet,1988,2(8613):737. [10] Mao M,Yu T,Hu J,et al. Dopamine D2 receptor blocker thioridazine induces cell death in human uterine cervical carcinoma cell line SiHa[J]. J Obstet Gynaecol Res,2015,41(8):1240-1245. doi: 10.1111/jog.12691 [11] Wang X,Shen Y,Wang S,et al. PharmMapper 2017 update:a web server for potential drug target identification with a comprehensive target pharmacophore database[J]. Nucleic Acids Res,2017,45(W1):W356-W360. doi: 10.1093/nar/gkx374 [12] Szklarczyk D,Gable A L,Lyon D,et al. STRING v11:protein-protein association networks with increased coverage,supporting functional discovery in genome-wide experimental datasets[J]. Nucleic Acids Res,2019,47(D1):D607-D613. doi: 10.1093/nar/gky1131 [13] Stelzer G,Rosen N,Plaschkes I,et al. The gene cards suite:From gene data mining to disease genome sequence analyses[J]. Curr Protoc Bioinformatics,2016,54:1.30.1-1.30.33. [14] Piñero J,Ramírez-Anguita J M,Saüch-Pitarch J,et al. The DisGeNET knowledge platform for disease genomics:2019 update[J]. Nucleic Acids Res,2020,48(D1):D845-D855. [15] Chin C H,Chen S H,Wu H H,et al. cytoHubba:identifying hub objects and sub-networks from complex interactome[J]. BMC Syst Biol,2014,8(Suppl 4):S11. [16] Wu T,Hu E,Xu S,et al. clusterProfiler 4.0:A universal enrichment tool for interpreting omics data[J]. Innovation (N Y),2021,2(3):100141. [17] Hartman Z,Geldenhuys W J,Agazie Y M. A specific amino acid context in EGFR and HER2 phosphorylation sites enables selective binding to the active site of Src homology phosphatase 2 (SHP2)[J]. J Biol Chem,2020,295(11):3563-3575. doi: 10.1074/jbc.RA119.011422 [18] Peng Z,Wang Q,Zhang Y,et al. EBP50 interacts with EGFR and regulates EGFR signaling to affect the prognosis of cervical cancer patients[J]. Int J Oncol,2016,49(4):1737-1745. doi: 10.3892/ijo.2016.3655 [19] Ding L and Zhang H. Circ-ATP8A2 promotes cell proliferation and invasion as a ceRNA to target EGFR by sponging miR-433 in cervical cancer[J]. Gene,2019,705:103-108. doi: 10.1016/j.gene.2019.04.068 [20] Mustafa H A,Albkrye A M S,AbdAlla B M,et al. Computational determination of human PPARG gene:SNPs and prediction of their effect on protein functions of diabetic patients[J]. Clin Transl Med,2020,9(1):7. [21] Wuertz B R,Darrah L,Wudel J,et al. Thiazolidinediones abrogate cervical cancer growth[J]. Exp Cell Res,2017,353(2):63-71. doi: 10.1016/j.yexcr.2017.02.020 [22] Noël J C,Bucella D,Fayt I,et al. Androgen receptor expression in cervical intraepithelial neoplasia and invasive squamous cell carcinoma of the cervix[J]. Int J Gynecol Pathol,2008,27(3):437-441. doi: 10.1097/PGP.0b013e318160c599 [23] Vannini F,Kashfi K,Nath N. The dual role of iNOS in cancer[J]. Redox Biol,2015,6:334-343. doi: 10.1016/j.redox.2015.08.009 [24] Topchieva L V,Balan O V,Korneva V A,et al. The nitric oxide metabolite level and NOS2 and NOS3 gene transcripts in patients with essential arterial hypertension[J]. Biology Bulletin,2020,47(3):247-252. doi: 10.1134/S1062359020010161 [25] Gray S,Axelsson B. The prevalence of deranged C-reactive protein and albumin in patients with incurable cancer approaching death[J]. PLoS One,2018,13(3):e0193693. doi: 10.1371/journal.pone.0193693 [26] Hoogenboezem E N,Duvall C L. Harnessing albumin as a carrier for cancer therapies[J]. Adv Drug Deliv Rev,2018,130:73-89. doi: 10.1016/j.addr.2018.07.011 [27] Chung S H,Wiedmeyer K,Shai A,et al. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer[J]. Cancer Res,2008,68(23):9928-9934. doi: 10.1158/0008-5472.CAN-08-2051 [28] Yu P,Wang Y,Li C,et al. Protective effects of downregulating estrogen receptor alpha expression in cervical cancer[J]. Anticancer Agents Med Chem,2018,18(14):1975-1982. [29] Chen Y,Gu Y,Gu Y,et al. Long noncoding RNA LINC00899/miR-944/ESR1 axis regulates cervical cancer cell proliferation,migration,and invasion[J]. J Interferon Cytokine Res,2021,41(6):220-233. doi: 10.1089/jir.2021.0023 [30] Xiao M,Feng Y,Cao G,et al. A novel MtHSP70-FPR1 fusion protein enhances cytotoxic T lymphocyte responses to cervical cancer cells by activating human monocyte-derived dendritic cells via the p38 MAPK signaling pathway[J]. Biochem Biophys Res Commun,2018,503(3):2108-2116. doi: 10.1016/j.bbrc.2018.07.167 [31] Li X,Chen L,Liang X J,et al. Annexin A5 protein expression is associated with the histological differentiation of uterine cervical squamous cell carcinoma in patients with an increased serum concentration[J]. Mol Med Rep,2012,6(6):1249-1254. doi: 10.3892/mmr.2012.1078 [32] Li X,Ma W,Wang X,et al. Annexin A5 overexpression might suppress proliferation and metastasis of human uterine cervical carcinoma cells[J]. Cancer Biomark,2018,23(1):23-32. doi: 10.3233/CBM-171040 [33] Youn M J,So H S,Cho H J,et al. Berberine,a natural product,combined with cisplatin enhanced apoptosis through a mitochondria/caspase-mediated pathway in HeLa cells[J]. Biol Pharm Bull,2008,31(5):789-795. doi: 10.1248/bpb.31.789 [34] Ng H H,Shen M,Samuel C S,et al. Relaxin and extracellular matrix remodeling:Mechanisms and signaling pathways[J]. Mol Cell Endocrinol,2019,487:59-65. doi: 10.1016/j.mce.2019.01.015 [35] Claesson-Welsh L,Welsh M. VEGFA and tumour angiogenesis[J]. J Intern Med,2013,273(2):114-127. doi: 10.1111/joim.12019 [36] Chuai Y,Rizzuto I,Zhang X,et al. Vascular endothelial growth factor (VEGF) targeting therapy for persistent,recurrent,or metastatic cervical cancer[J]. Cochrane Database Syst Rev,2021,2021(3):CD013348. [37] Li J,Yao Q Y,Xue J S,et al. Dopamine D2 receptor antagonist sulpiride enhances dexamethasone responses in the treatment of drug-resistant and metastatic breast cancer[J]. Acta Pharmacol Sin,2017,38(9):1282-1296. doi: 10.1038/aps.2017.24 -






 下载:
下载: