Screening and Analysis of Pathogenic Genes in Families with Atrial Septal Defects
-
摘要:
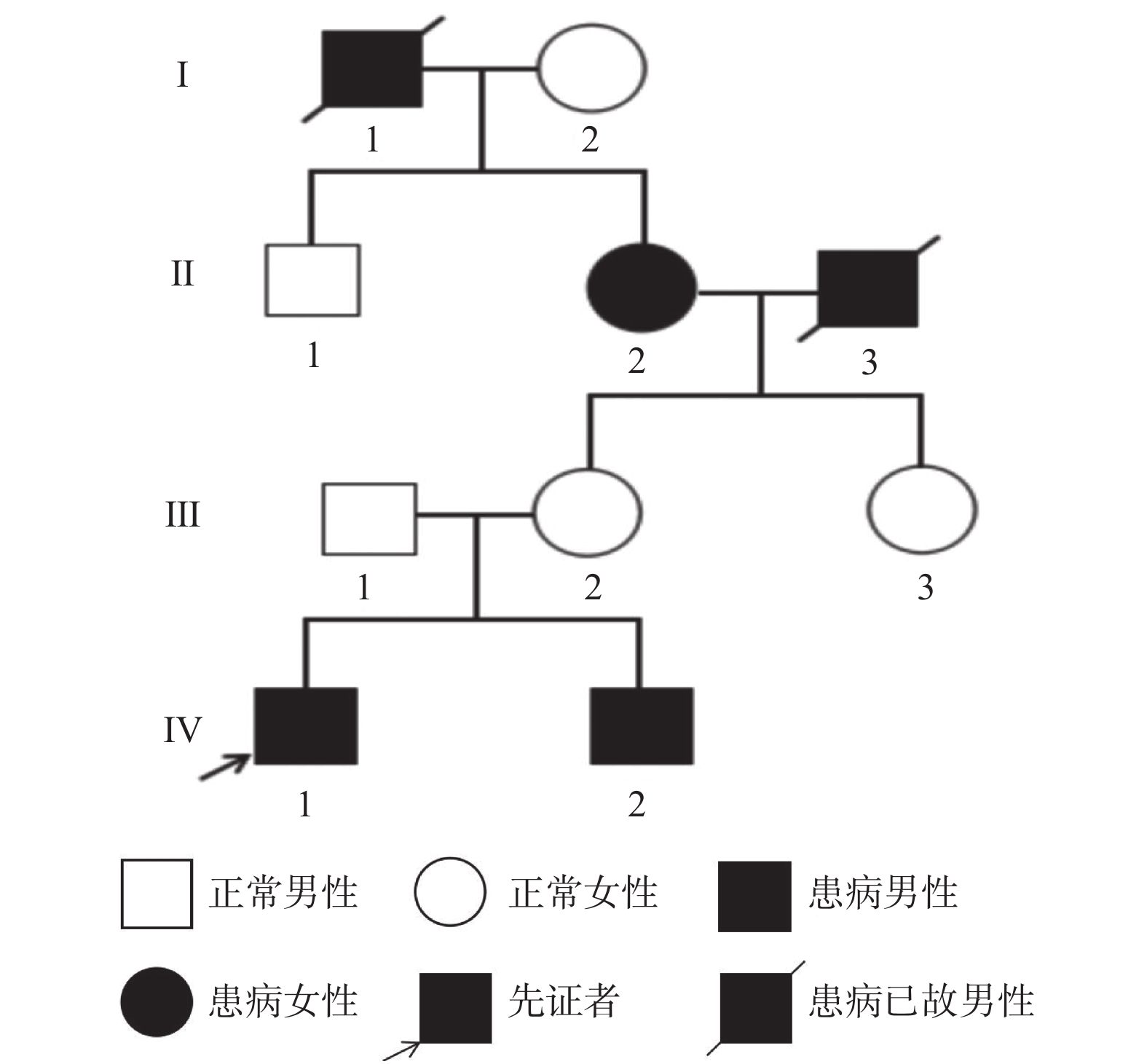
目的 通过对一个房间隔缺损先证者的四代家系进行致病突变筛查,利用全外显子测序及Sanger测序等分析基因型和表型之间的关系。 方法 回顾性分析在云南省第一人民医院心脏大血管外科就诊的1例房间隔缺损患儿的临床资料,并采用全外显子测序技术对患儿及其弟弟、母亲进行遗传学检测,结合生物信息学分析,寻找导致该家系致病的遗传学病因。同时,对在该家系中发现的尾型同源盒基因4(Caudal Homebox Gene,CDX4)基因变异,进行散发ASD患者及健康人群的筛查。 结果 患儿心脏超声结果及术中所见符合ASD诊断。基因检测提示患儿存在CDX4基因c.C233T纯合变异,其母亲携带该基因的杂合变异,其弟弟携带该基因纯合变异。CDX4基因第1外显子第233号密码子由C突变为T(c.C233T),导致第78号氨基酸由脯氨酸变为亮氨酸(p.P78L)。在198例散发性房间隔缺损患者中,5例患者存在该基因变异,而265例健康人群中未发现该基因变异。采用Fischer精确检验分析发现CDX4基因c.C233T纯合变异与先天性心脏病相关(P = 0.014)。 结论 CDX4基因c.C233T(p.P78L)纯合突变很可能是该家系的致病变异。 Abstract:Objective To screen the pathogenic mutations in the four generations of a proband with atrial septal defect, so as to analyze the relationship between genotype and phenotype by whole exon sequencing and Sanger sequencing. Methods Retrospectic analysis of the clinical data on a child with atrial septal defects treated in the Department of Cardiovascular Surgery, the First People’ s Hospital of Yunnan Province was conducted in tandem with genetic tests for the sick child, his younger brother and mother by means of whole-exome sequencing to find out the genetic causes of the family in combination with bioinformatics analysis. Meanwhile, sporadic ASD patients and healthy people were screened for the Caudal Homebox Gene 4 (CDX4) mutation found in the family. Results The cardiac ultrasound results of the sick child and the intraoperative findings conformed with ASD diagnosis. The genetic tests suggested that CDX4 gene c.C233T homozygous mutation occurred to the child, his mother carries heterozygous mutation of the gene and his younger brother homozygous mutation of it. Codon 233 of exon 1 of CDX4 gene mutated from C to T (c.C233T), leading to conversion of amino acid 78 from proline to leucine (p.P78L). Multi-species comparison showed that the locus was highly conserved. The three software programs PROVEAN, Mutation Taster and Mutation Assessor suggested that the mutation was harmful. Of the 198 patients with sporadic atrial septal defects, 5 suffered this gene mutation, but that was not found in the 265 healthy people. According to the Fischer’s Exact Test, CDX4 gene c.C233T homozygous mutation was associated with congenital heart diseases (P = 0.014). Conclusion CDX4 gene c.C233T (p.P78L) homozygous mutation is the highly likely the pathogenic mutation of the family. -
Key words:
- Atrial Septal Defect /
- Whole exon sequencing /
- CDX4 /
- Gene mutation
-
表 1 Sanger测序人群验证及变异的致病性分析结果
Table 1. Population validation and pathogenicity analysis results of Sanger sequencing
基因
类型房间隔缺损患者 健康人群 P n 百分比(%) n 百分比(%) CT 5 2.52 0 0 0.014 TT 193 97.48 265 100 -
[1] Lindsey J B,Hillis L D. Clinical update:atrial septal defect in adults[J]. The Lancet,2007,369(9569):1244-1246. doi: 10.1016/S0140-6736(07)60576-5 [2] Humenberger M,Rosenhek R,Gabriel H,et al. Benefit of atrial septal defect closure in adults:impact of age[J]. European Heart Journal,2011,32(5):553-560. doi: 10.1093/eurheartj/ehq352 [3] Benjamin E J,Muntner P,Alonso A,et al. Heart disease and stroke statistics—2019 update:a report from the American Heart Association[J]. Circulation,2019,139(10):e56-e528. [4] Buijtendijk M F J,Barnett P,van den Hoff M J B,et al. Development of the human heart[J]. American Journal of Medical Genetics Part C-seminars In Medical Genetics,2020,184(1):7-22. doi: 10.1002/ajmg.c.31778 [5] Aoki H, Horie M. Electrical disorders in atrial septal defect: genetics and heritability[J]. Journal of Thoracic Disease, 2018, 10(Suppl 24): S2848. [6] Wessels M W,Willems P J. Genetic factors in non-syndromic congenital heart malformations[J]. Clinical Genetics,2010,78(2):103-123. doi: 10.1111/j.1399-0004.2010.01435.x [7] Fan D,Pang S,Chen J,et al. Identification and functional study of GATA4 gene regulatory variants in atrial septal defects[J]. BMC Cardiovasc Disord,2021,21(1):321. [8] Li Z,Huang J,Liang B,et al. Copy number variations in the GATA4,NKX2-5,TBX5,BMP4 CRELD1,and 22q11.2 gene regions in Chinese children with sporadic congenital heart disease[J]. J Clin Lab Anal,2019,33(2):e22660. [9] Huang S,Wu Y,Chen S,et al. Novel insertion mutation (Arg1822_Glu1823dup) in MYH6 coiled-coil domain causing familial atrial septal defect[J]. Eur J Med Genet,2021,64(11):104314. [10] Chawengsaksophak K,de Graaff W,Rossant J,et al. Cdx2 is essential for axial elongation in mouse development[J]. Proceedings of the National Academy of Sciences,2004,101(20):7641-7645. doi: 10.1073/pnas.0401654101 [11] Freund J N,Duluc I,Reimund J M,et al. Extending the functions of the homeotic transcription factor Cdx2 in the digestive system through nontranscriptional activities[J]. World Journal of Gastroenterology:WJG,2015,21(5):1436-1443. doi: 10.3748/wjg.v21.i5.1436 [12] Lengerke C,Wingert R,Beeretz M,et al. Interactions between Cdx genes and retinoic acid modulate early cardiogenesis[J]. Developmental Biology,2011,354(1):134-142. doi: 10.1016/j.ydbio.2011.03.027 [13] Davidson A J,Ernst P,Wang Y,et al. cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes[J]. Nature,2003,425(6955):300-306. doi: 10.1038/nature01973 [14] Skromne I,Thorsen D,Hale M,et al. Repression of the hindbrain developmental program by Cdx factors is required for the specification of the vertebrate spinal cord[J]. Development,2007,134(11):2147-2158. doi: 10.1242/dev.002980 [15] Cho SW,Kim HK,Sung JH,et al. Stage specific transcriptome profiles at cardiac lineage commitment during cardiomyocyte differentiation from mouse and human pluripotent stem cells[J]. BMB Rep,2021,54(9):464-469. [16] Louryan S,Vanmuylder N. Contributions of embryology and comparative anatomy for teaching of cranial nerves[J]. Morphologie,2018,102(337):111-121. [17] Hayward A G,Joshi P,Skromne I. Spatiotemporal analysis of zebrafish hox gene regulation by Cdx4[J]. Developmental Dynamics,2015,244(12):1564-1573. doi: 10.1002/dvdy.24343 [18] Hunt P,Krumlauf R. Deciphering the Hox code:clues to patterning branchial regions of the head[J]. Cell,1991,66(6):1075-1078. doi: 10.1016/0092-8674(91)90029-X [19] Foley T E,Hess B,Savory,J G A,et al. Role of Cdx factors in early mesodermal fate decisions[J]. Development,2019,146(7):dev170498. doi: 10.1242/dev.170498 [20] Su W,Zhu P,Wang R,et al. Congenital heart diseases and their association with the variant distribution features on susceptibility genes[J]. Clin Genet,2017,91(3):349-354. [21] Hofbauer P,Jahnel SM,Papai N,et al. Cardioids reveal self-organizing principles of human cardiogenesis[J]. Cell,2021,184(12):3299-3317. doi: 10.1016/j.cell.2021.04.034 [22] Lohnes D. The Cdx1 homeodomain protein:an integrator of posterior signaling in the mouse[J]. Bioessays,2003,25(10):971-980. doi: 10.1002/bies.10340 [23] Pilon N,Oh K,Sylvestre J R,et al. Cdx4 is a direct target of the canonical Wnt pathway[J]. Developmental Biology,2006,289(1):55-63. doi: 10.1016/j.ydbio.2005.10.005 [24] Reichman DE,Park L,Man L,et al. Wnt inhibition promotes vascular specification of embryonic cardiac progenitors[J]. Development,2018,145(1):dev.159905. [25] Miao S,Zhao D,Wang X,et al. Retinoic acid promotes metabolic maturation of human Embryonic Stem Cell-derived Cardiomyocytes[J]. Theranostics,2020,10(21):9686-9701. doi: 10.7150/thno.44146 [26] Xavier-Neto J,Sousa Costa A M,Figueira A C,et al. Signaling through retinoic acid receptors in cardiac development:doing the right things at the right times[J]. Biochimica et Biophysica Acta,2015,1849(2):94-111. [27] Xiaoqian,Zhang,Henghua,et al. Differentiation and characterization of rhesus monkey atrial and ventricular cardiomyocytes from induced pluripotent stem cells[J]. Stem Cell Research,2017,20:21-29. doi: 10.1016/j.scr.2017.02.002 -






 下载:
下载: