Significance of CXCL10 as a Biomarker of Liver Cirrhosis
-
摘要:
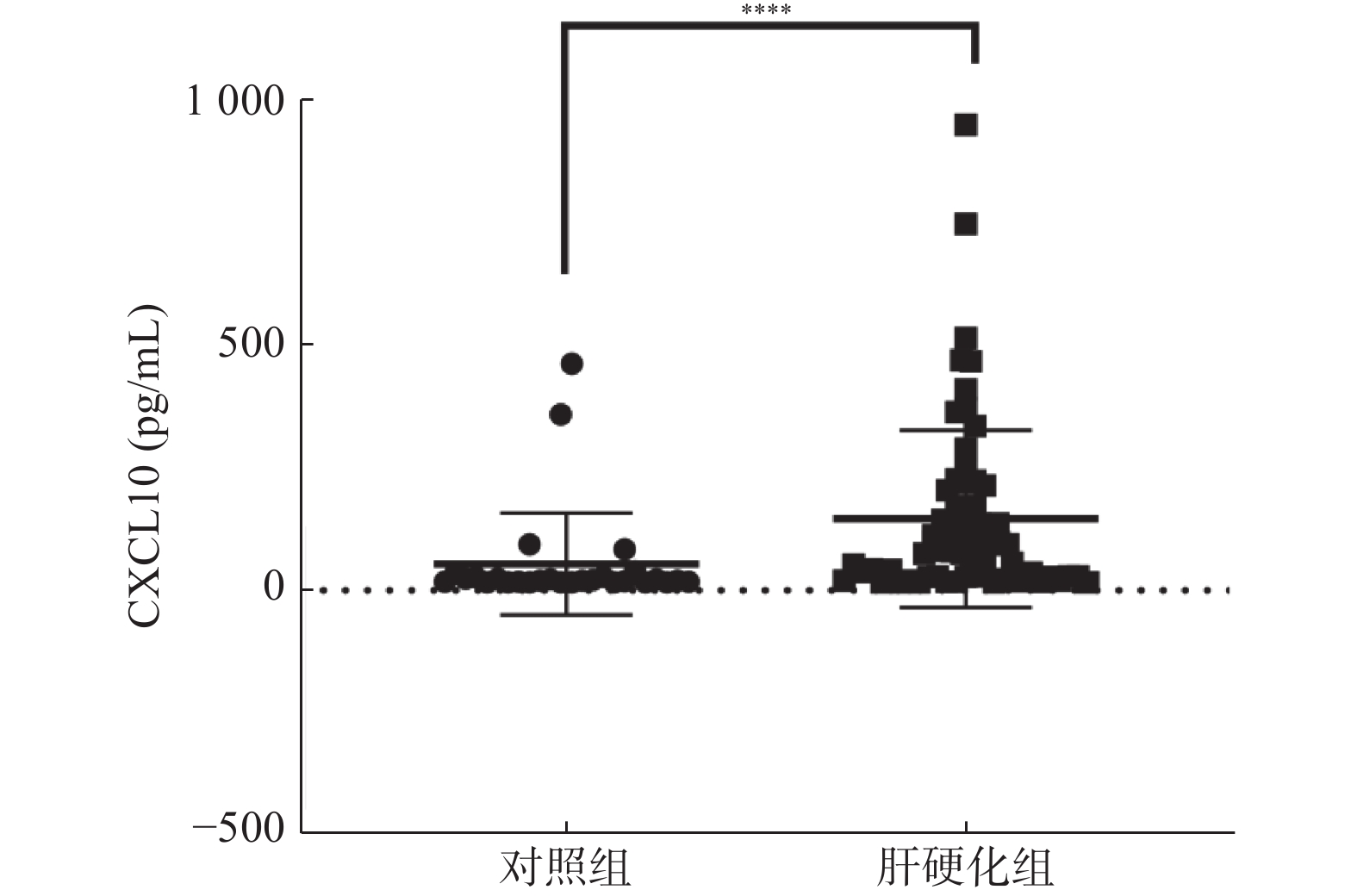
目的 探讨CXCL10作为一种肝硬化无创生物标志物的潜力及其在肝纤维化进展中的机制。 方法 收集2021年3~8月期间昆明医科大学第二附属医院肝硬化患者(n = 64)和健康体检者(n = 64)EDTA-K2抗凝管外周血标本,严格记录患者的基本信息和实验室检查结果。ELISA检测血浆中CXCL10浓度。分析CXCL10表达水平与生化检验结果的关系。用ROC曲线分析血浆CXCL10水平对肝硬化的预测价值。 结果 肝硬化患者血浆CXCL10浓度显著高于健康体检者,差异有统计学意义(P < 0.0001)。肝硬化患者血浆CXCL10水平与检验结果如ALT、AST、PT、APTT、DD成正相关关系(P < 0.05)。CXCL10的ROC曲线下面积为0.8264,具有较好的诊断效能。 结论 CXCL10可作为肝硬化的一种无创生物标志物,也是肝硬化潜在的治疗靶点。 Abstract:Objective To investigate the potential of CXCL10 as a noninvasive biomarker of liver cirrhosis and its mechanism in the progression of liver fibrosis. Methods Peripheral blood samples of EDTA-K2 anticoagulant tubes were collected from cirrhotic patients (n = 64) and healthy subjects (n = 64) in the Second Affiliated Hospital of Kunming Medical University from March to August 2021, and the basic information and laboratory examination results of the patients were strictly recorded. CXCL10 concentration in plasma was determined by ELISA. The relationship between CXCL10 expression level and biochemical test results was analyzed. ROC curve was used to analyze the predictive value of plasma CXCL10 levels for cirrhosis. Results The plasma CXCL10 concentration in patients with cirrhosis was significantly higher than that in healthy subjects, and the difference was statistically significant (P < 0.0001). Plasma CXCL10 levels were positively correlated with ALT, AST, PT, APTT and DD in patients with cirrhosis (P < 0.05). The area under ROC curve of CXCL10 is 0.8264, which has good diagnostic performance. Conclusion CXCL10 can be used as a non-invasive biomarker and a potential therapeutic target for liver cirrhosis. -
Key words:
- Liver fibrosis /
- CXCL10 /
- Liver cirrhosis
-
表 1 肝硬化患者的临床资料[
$ \bar x \pm s $ /M(P25,P75)]Table 1. Clinical data of patients with cirrhosis [
$ \bar x \pm s $ /M(P25,P75)]特征 数值 年龄(岁) 64 ± 11.52 性别(男/女) 46/18 ALT(U/L) 33(21,56.75) AST(U/L) 47(32,74.25) ALP(U/L) 98(80,159) GGT(U/L) 46(31.5,89.5) TBA(μmol/L) 43.55(19,97.43) TBIL(μmol/L) 30.55(17.03,70.9) DBIL(μmol/L) 14.10(6.9,38.4) IBIL(μmol/L) 15.55(7.8,25.93) PT (s) 16.94 ± 2.97 APTT(s) 44.23 ± 8.03 TT(s) 18.1(16.88,19.1) FIB(g/L) 1.86(1.53,2.56) DD(μg/mL) 2.68(1.73,4.79) 表 2 肝硬化患者血浆CXCL10浓度与肝功生化指标关系
Table 2. Relationship between plasma CXCL10 concentration and liver function biochemical indexes in patients with cirrhosis
项目 r P ALT(U/L) 0.4261 0.0004* AST(U/L) 0.36 0.0035* ALP(U/L) 0.01119 0.9354 GGT(U/L) 0.1621 0.2007 TBA(μmol/L) 0.2065 0.1074 TBIL(μmol/L) −0.03392 0.7902 DBIL(μmol/L) −0.00793 0.9512 IBIL(μmol/L) −0.1005 0.4372 *P < 0.05。 表 3 肝硬化患者血浆CXCL10浓度与凝血指标关系
Table 3. Relationship between plasma CXCL10 concentration and coagulation indexes in patients with cirrhosis
项目 r P PT 0.4085 0.0008* APTT 0.2879 0.0211* TT 0.03217 0.8709 FIB −0.01198 0.9264 DD 0.3279 0.012* *P < 0.05。 表 4 CXCL10相关影响因素的线性回归分析
Table 4. Linear regression analysis of CXCL10 related influencing factors
变量 β t P ALT −0.30 −0.148 0.883 AST 0.108 0.466 0.644 ALP −0.031 −0.112 0.912 GGT 0.033 0.129 0.898 TBA 0.235 1.201 0.238 TBIL −0.177 −0.527 0.601 DBIL −0.009 −0.054 0.957 IBIL −0.127 −0.466 0.644 PT 0.129 0.548 0.587 APTT 0.020 0.077 0.939 TT 0.312 1.383 0.176 FIB 0.164 0.810 0.423 DD 0.309 1.922 0.043* *P < 0.05。 表 5 高表达CXCL10组和低表达CXCL10组实验室指标差异分析[
$ \bar x \pm s $ /M(P25,P75)]Table 5. Difference analysis of laboratory indicators between high and low expression groups of CXCL10 [
$ \bar x \pm s $ /M(P25,P75)]指标 低表达CXCL10(n = 31) 高表达CXCL10(n = 33) Z/t P ALT(U/L) 26(9,127) 46(20,148) −3.529 0.0003* AST(U/L) 37(17,137) 54(21,141) −2.486 0.0123* LPS(U/L) 98(54,441) 97(43,859) −0.800 0.9633 GGT(U/L) 39(21,264) 51(26,795) −1.559 0.1338 TBA(μ
mol/L)32.6(7.8,116.1) 50.8(8.1,408.3) −2.606 0.5693 TBIL(µmol/L) 31.7(9.8,404.5) 25.6(12.6,550) −0.013 0.9920 DBIL(µmol/L) 15(3.9,291.2) 12.7(6.4,440.9) −0.336 0.9137 IBIL(µmol/L) 16.7(4.3,113.31) 11.2(6.1,164.7) −0.262 0.5639 PT(s) 16.04 ± 0.44 17.58 ± 0.57 2.465 0.0165* APTT(s) 41.98 ± 1.28 46.19 ± 1.54 2.253 0.0278* TT(s) 18.22 ± 0.34 18.10 ± 0.43 0.1522 0.8795 FIB(g/L) 1.74(0.86,3.14) 1.89(0.85,3.51) −0.470 0.9582 DD(μg/mL) 1.73(0.33,7.43) 3.81(0.44,12.22) −2.767 0.0041* *P < 0.05。 -
[1] Zoubek M E,Trautwein C,Strnad P. Reversal of liver fibrosis:From fiction to reality[J]. Best Pract Res Clin Gastroenterol,2017,203(3):12-41. [2] Moon A M,Singal A G,Elliot B. Contemporary epidemiology of chronic liver disease and cirrhosis - science direct[J]. Clinical Gastroenterology and Hepatology,2020,18(12):50-66. [3] Simon T G,Roelstraete B,Hartjes K,et al. Non-alcoholic fatty liver disease in children and young adults is associated with increased long-term mortality[J]. Journal of Hepatology,2021,20(5):135-141. [4] Campos-Murgu í a A,Román-Calleja B,Toledo-Coronado I V,et al. Liver fibrosis in patients with metabolic associated fatty liver disease is a risk factor for adverse outcomes in COVID-19[J]. Digestive and Liver Disease,2021,48(21):48-56. [5] Caviglia J M,Yan J,Jang M K,et al. MicroRNA-21 and dicer are dispensable for hepatic stellate cell activation and the development of liver fibrosis[J]. Hepatology,2017,67(Suppl.1):65-72. [6] Lee Y S,Kim S Y,Ko E,et al. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells[J]. Scientific Reports,2017,7(1):30-45. [7] Holt A P,Haughton E L,Lalor P F,et al. Liver myofibroblasts regulate Infiltration and positioning of lymphocytes inhuman liver[J]. Gastroenterology,2009,136(2):5-14. doi: 10.1053/j.gastro.2008.09.006 [8] Barrow F,Khan S,Fredrickson G,et al. Microbiota‐Driven Activation of intrahepatic B Cells aggravates nonalcoholic steatohepatitis through innate and adaptive signaling[J]. Hepatology,2018,74(23):84-96. [9] Lafoz E,Ruart M,Anton A,et al. The endothelium as a driver of liver fibrosis and regeneration[J]. Cells,2020,9(4):29-35. [10] Barrow F,Khan S,Fredrickson G,et al. Exosomes from activated hepatic stellate cells contain GLUT1 and PKM2:a role for exosomes in metabolic switch of liver nonparenchymal cells[J]. The FASEB Journal,2019,33(7):41-56. [11] Motonobu,Watanabe,Soichiro,et al. Platelets contribute to the reduction of liver fibrosis in mice[J]. Journal of Gastroenterology & Hepatology,2009,21(17):23-32. [12] Ding B S,Ca O Z,Lis R,et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis[J]. Nature,2014,505(7481):97-102. doi: 10.1038/nature12681 [13] Tannapfel A,Dienes H P,Lohse A W. The indications for liver biopsy[J]. Deutsches Ä rzteblatt International,2012,109(27-28):477-483. [14] Song Z Y,Ke-Bang W U,Li-Li W U,et al. Research progress on rapid serological assays for diagnosis of Japanese Encephalitis B[J]. China Animal Husbandry & Veterinary Medicine,2015,45(23):69-75. [15] Wang R,Ding Q,Yaqoob U,Assuncao T D,et al. Non-invasive assessment of hepatic steatosis:Prospective comparison of the accuracy of imaging examinations[J]. Journal of Hepatology,2010,52(4):79-85. [16] Kim S U,Seo Y S,Cheong J Y,et al. Factors that affect the diagnostic accuracy of liver fibrosis measurement by Fibroscan in patients with chronic hepatitis B[J]. Alimentary Pharmacology & Therapeutics,2010,32(3):498-505. [17] Wang W J, Xiao P, Xu H Q, et al. Growing burden of alcoholic liver disease in China: A review [J]. 世界胃肠病学杂志: 英文版, 2019, 25(12): 12.Wang W J,Xiao P,Xu H Q,et al. Growing burden of alcoholic liver disease in China:A review[J]. World Journal Gastroenterology,2019,25(12):12. [18] Ahmed Z,Ahmed U,Walayat S,et al. Liver function tests in identifying patients with liver disease[J]. Clinical and Experimental Gastroenterology,2018,11(6):1-7. [19] Liu Z,Zhang Z,Huang M,et al. Taurocholic acid is an active promoting factor,not just a biomarker of progression of liver cirrhosis:evidence from a human metabolomic study and in vitro experiments[J]. Bmc Gastroenterology,2018,18(1):112-116. doi: 10.1186/s12876-018-0842-7 -






 下载:
下载: