Scutellarin Ameliorates Liver Fibrosis in Nonalcoholic Fatty Liver Disease by Inhibiting NOX Expression
-
摘要:
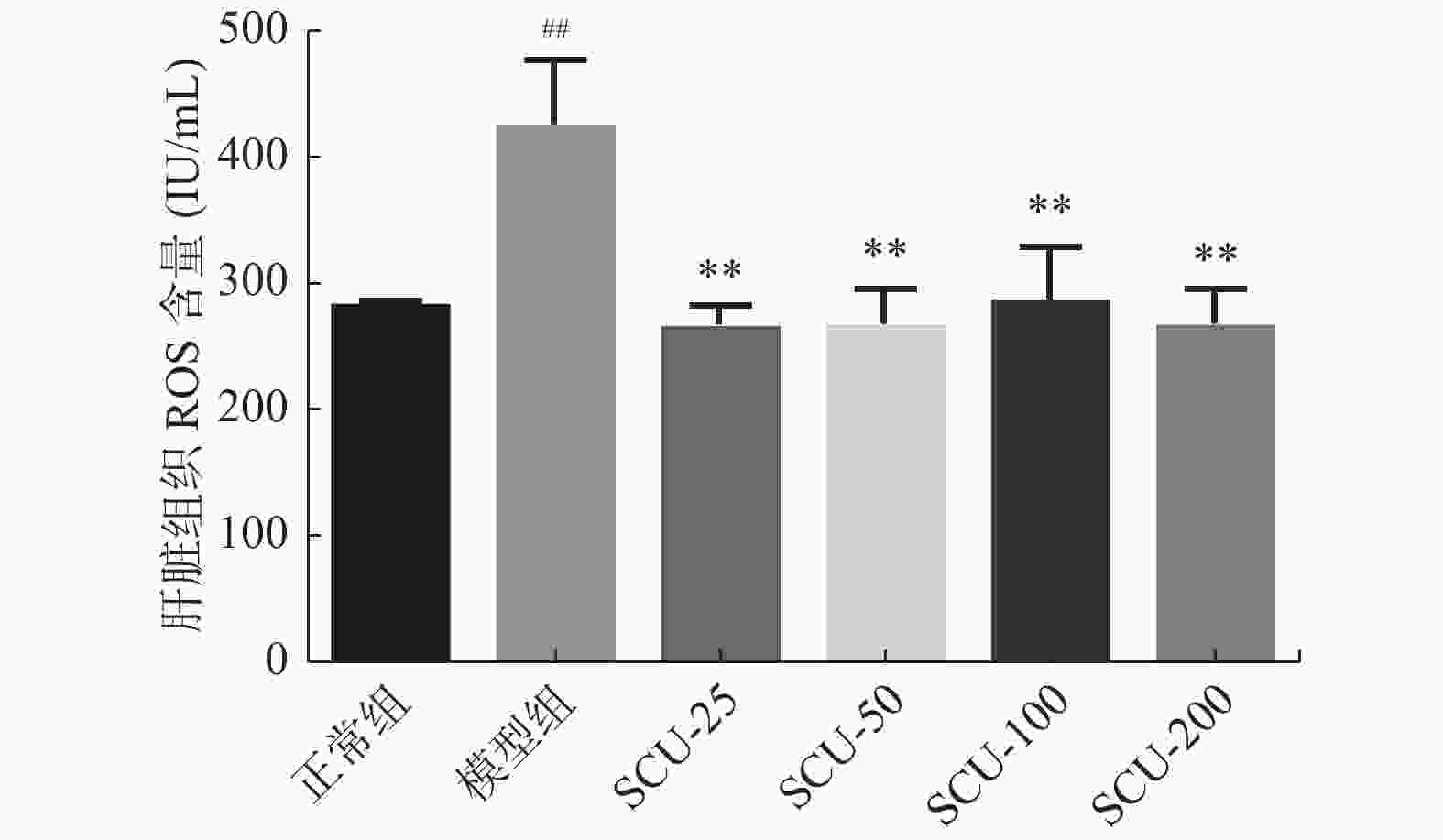
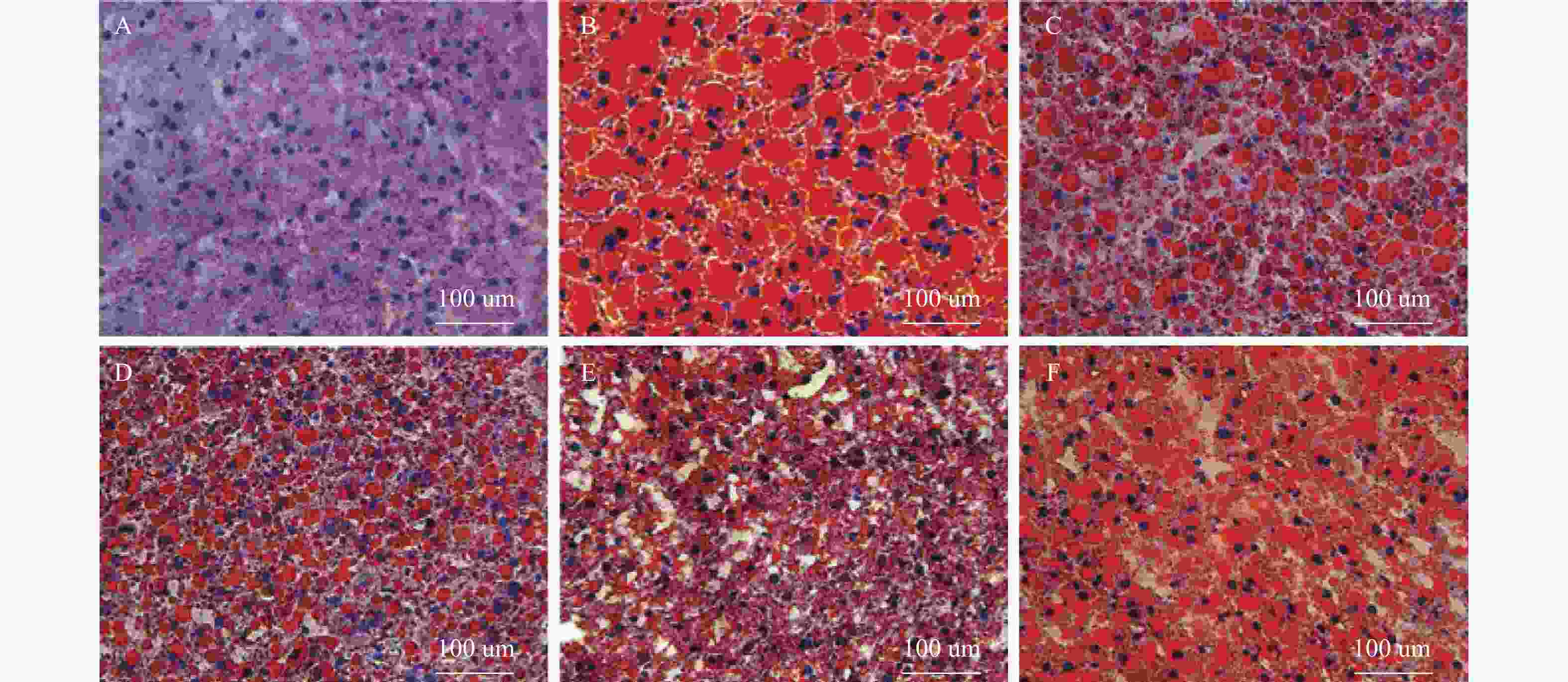
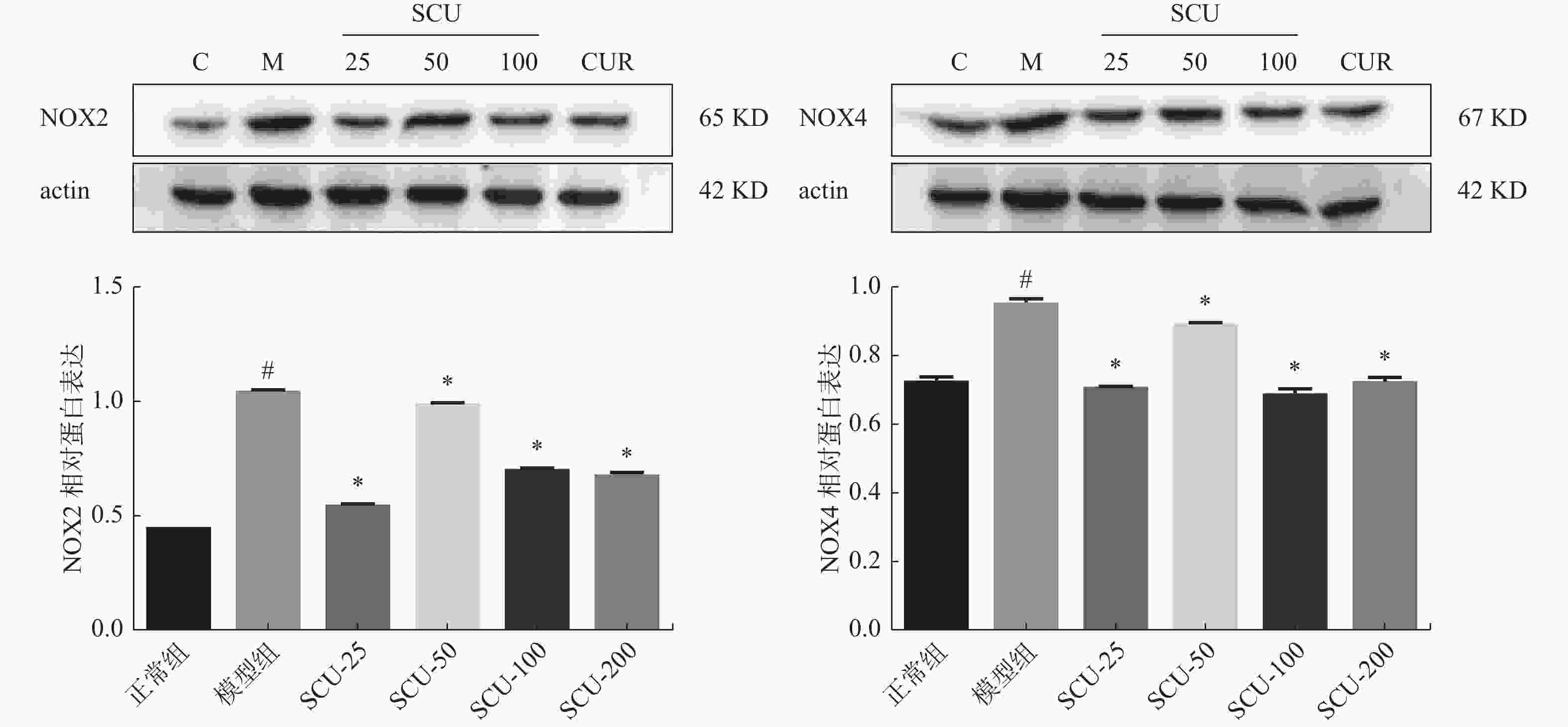
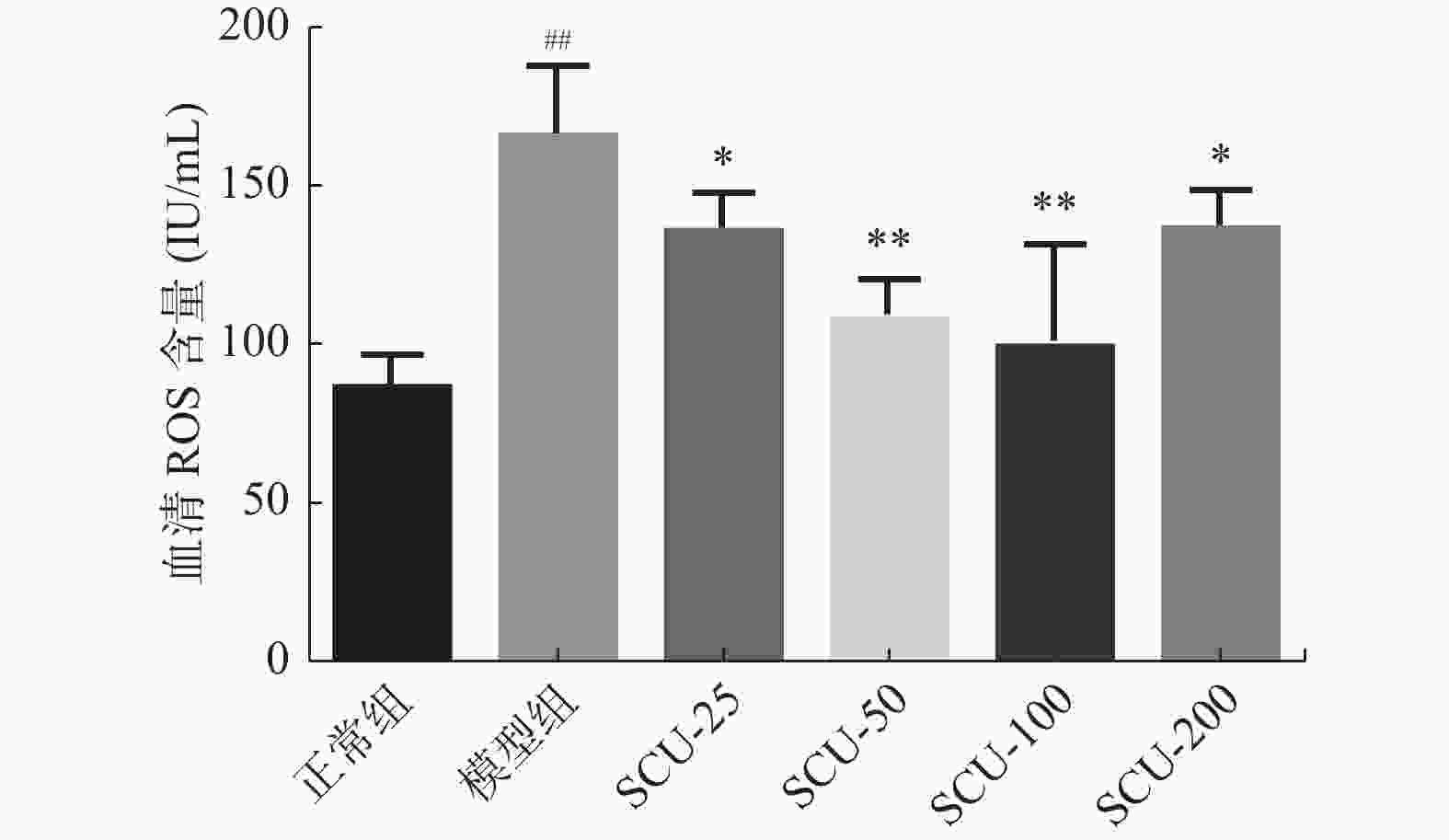
目的 探讨灯盏乙素(SCU)抑制NOX(NOX2、NOX4)的表达,改善高脂高糖诱导的非酒精性脂肪性肝病(NAFLD)肝脏纤维化程度。 方法 60只大鼠随机分为:正常组、模型组、SCU-25低剂量治疗组[25 mg/(kg·d)]、SCU-50中剂量治疗组[50 mg/(kg·d)]SCU-100高剂量治疗组[100 mg/(kg·d)]和姜黄素阳性药物治疗组[200 mg/(kg·d)],每组10只。正常组给予普通饲料喂养24+8周,其余各组采用高脂高糖饲料喂养SD大鼠24周建立NAFLD动物模型后,给予不同浓度的SCU和姜黄素灌胃治疗8周。治疗完成后,称取大鼠的体重,麻醉状态下取大鼠血清和肝脏组织,称取肝脏的重量,计算肝脏指数;通过ELISA法检测血清和组织中ROS的含量;采用油红“O”染色检测肝脏组织脂质沉积,Masson染色检测肝脏组织的纤维化程度;Western blot和免疫组织化学法检测肝脏组织中NOX2、NOX4蛋白的表达。 结果 模型组大鼠体重、肝重及肝脏指数增加(P < 0.01),不同剂量的SCU和姜黄素治疗后体重、肝重及肝脏指数降低,SCU-100高剂量治疗组和姜黄素阳性药物治疗组对体重和肝重降低的影响较为明显( P < 0.01)。模型组血清和肝脏组织ROS含量增加( P < 0.01),不同剂量的SCU和姜黄素治疗后ROS含量降低( P < 0.05)。在肝脏组织中,模型组出现大量的脂质沉积和组织的纤维化表现,不同剂量的SCU和姜黄素治疗后,脂质沉积减少,肝脏的纤维化程度改善。在肝脏组织细胞中,模型组的NOX2、NOX4蛋白表达上调( P < 0.05),不同剂量的SCU和姜黄素治疗后NOX2、NOX4的蛋白表达下调( P < 0.05),但剂量依赖不明显。 结论 SCU可能通过抑制肝脏NOX2、NOX4蛋白的表达,降低ROS的产生,从而改善NAFLD的肝脏纤维化程度。 Abstract:Objective To investigate the effect of scutellarin (SCU) on the expression of NOX (NOX2, NOX4) and the degree of liver fibrosis induced by high fat and high sugar in non-alcoholic fatty liver disease (NAFLD). Methods Sixty rats were randomly divided into normal group, model group, SCU-25 lower dose treatment group[25 mg/(kg·d), SCU-50 dose in the treatment group[50 mg/(kg·d)], SCU-100 high dose treatment group [100 mg/(kg·d) and curcumin positive drug treatment group[200 mg/(kg·d)], 10 in each group. The normal group was fed with ordinary diet for 24+8 weeks, and the other groups were fed with high-fat and high- sugar diet for 24 weeks to establish NAFLD animal model, and then given Different concentrations of SCU and curcumin intragastric treatment for 8 weeks. After the treatment, the weight of the rats was measured, and the serum samples and liver tissues of the rats were collected under anesthesia. The weight of theliver was measured and the liver index was calculated. ROS content in serum and tissues was detected by ELISA. Using oil red "O" Dyeing, detection of liver lipid deposition and Masson staining to detect the degree of fibrosis of liver tissue; Western blot and immunohistochemistry were used to detect the expression of NOX2 and NOX4 proteins in liver tissues. Results Body weight, liver weight and liver index increased in model group ( P < 0.01), body weight, liver weight and liver index decreased after different doses of SCU and curcumin treatment group, while body weight and liver weight were significantly decreased in SCU-100 high-dose treatment group and curcumin positive drug treatment group ( P < 0.01). ROS content in serum and liver tissues of model group was significantly increased ( P < 0.01), and ROS content was decreased after treatment with different doses of SCU and curcumin ( P < 0.05). In the liver tissue, the model group showed a large amount of lipid deposition and tissue fibrosis. After treatment with different doses of SCU and curcumin, the lipid deposition was reduced and the degree of liver fibrosis wasimproved. In liver tissue cells, the protein expressions of NOX2 and NOX4 were up-regulated in the model group ( P < 0.05), but down-regulated in differentdoses of SCU and curcumin ( P < 0.05), but not dose-dependent. Conclusion SCU may reduce ROS production by inhibiting the expression of NOX2 and NOX4 proteins in liver, thus alleviating the degree of liver fibrosis in NAFLD. -
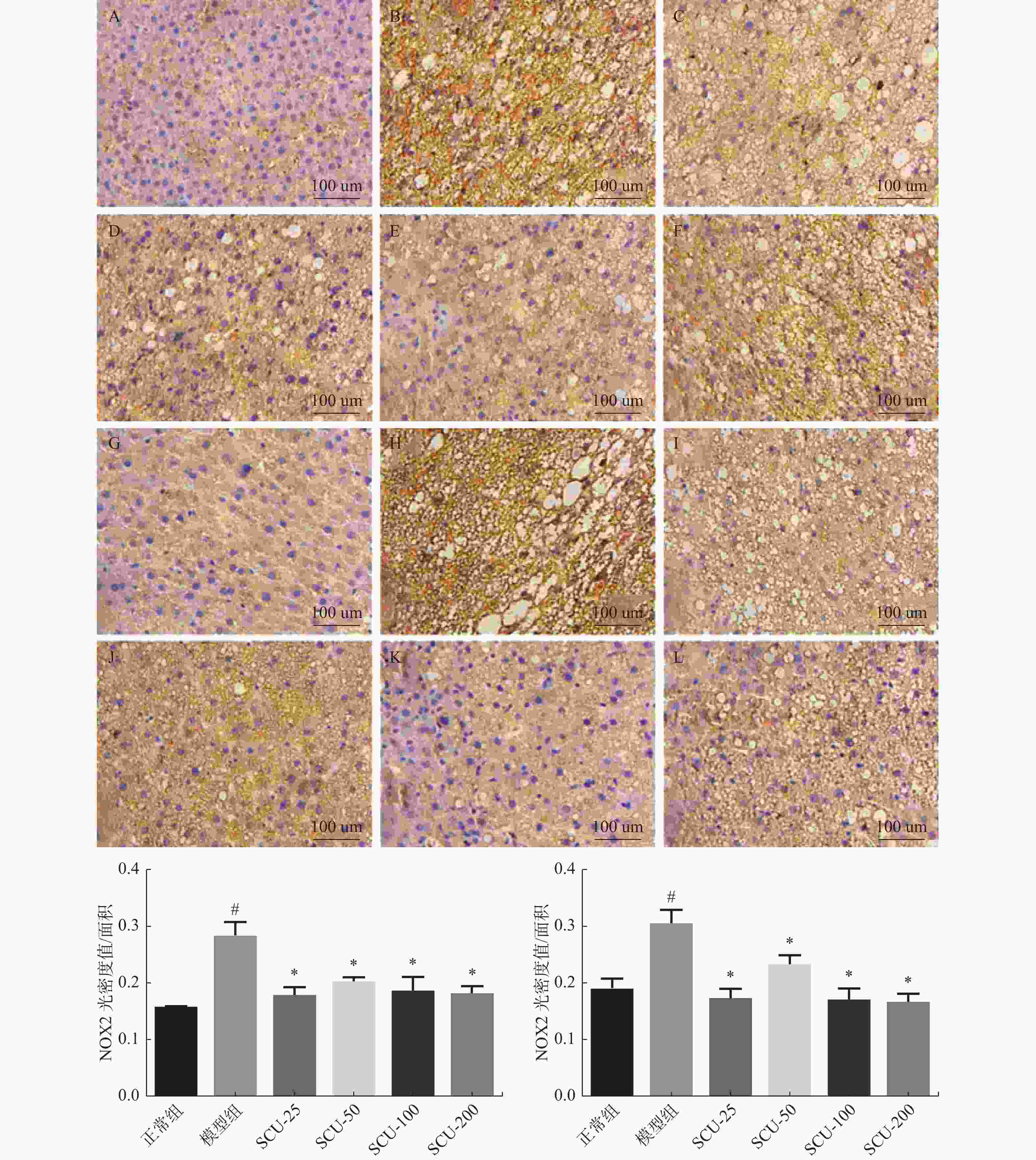
图 6 肝脏组织中NOX2、NOX4蛋白的表达(×400)
A:正常组NOX2表达;B:模型组NOX2表达;C:SCU-25 NOX2表达;D:SCU-50 NOX2表达;E:SCU-100 NOX2表达;F:CUR-200 NOX2表达;G:正常组NOX4表达;H:模型组NOX4表达;I:SCU-25 NOX4表达;J:SCU-50 NOX4表达;K:SCU-100 NOX4表达;L:CUR-200 NOX4表达。与正常组比较,#P < 0.05,与模型组比较,*P < 0.05。
Figure 6. Expression of NOX2 and NOX4 proteins in liver tissue (×400)
表 1 大鼠体重、肝重及肝脏指数(
$\bar x \pm s $ )Table 1. Body weight,liver weight and liver index of rats (
$\bar x \pm s $ )项目 正常组 模型组 Scu-25 Scu-50 Scu-100 Cur-200 体重(g) 547.60 ± 38.71 888.00 ± 75.16 ## 852.40 ± 56.86 801.40 ± 19.51 702.00 ± 38.39 ** 725.60 ± 45.24 ** 肝重(g) 14.03 ± 1.36 39.04 ± 3.09 ## 35.80 ± 7.01 30.70 ± 3.23 ** 28.03 ± 2.24 ** 26.43 ± 2.03 ** 肝脏指数 2.571 ± 0.268 4.415 ± 0.381 ## 4.184 ± 0.662 3.825 ± 0.333 4.016 ± 0.005 3.639 ± 0.078 * 与正常组比较, ## P < 0.01,与模型组比较, *P < 0.01, **P < 0.01。 -
[1] Liu Y,Nong L,Jia Y,et al. Aspirin alleviates hepatic fibrosis by suppressing hepatic stellate cells activation via the TLR4/NF-kappaB pathway[J]. Aging (Albany NY),2020,12(7):6058-6066. doi: 10.18632/aging.103002 [2] Zhao Z B,Ji K,Shen X Y,et al. Di(2-ethylhexyl) phthalate promotes hepatic fibrosis by regulation of oxidative stress and inflammation responses in rats[J]. Environ Toxicol Pharmacol,2019,68(5):109-119. [3] Du J J,Sun J C,Li N,et al. beta-Arrestin2 deficiency attenuates oxidative stress in mouse hepatic fibrosis through modulation of NOX4[J]. Acta Pharmacol Sin,2021,42(7):1090-1100. doi: 10.1038/s41401-020-00545-9 [4] Wang T,Zhou X,Kuang G,et al. Paeoniflorin modulates oxidative stress,inflammation and hepatic stellate cells activation to alleviate CCl4-induced hepatic fibrosis by upregulation of heme oxygenase-1 in mice[J]. J Pharm Pharmacol,2021,73(3):338-346. doi: 10.1093/jpp/rgaa042 [5] Liang S,Kisseleva T,Brenner D A. The role of NADPH oxidases (NOXs) in liver fibrosis and the activation of myofibroblasts[J]. Front Physiol,2016,7(2):17. [6] 王银辉,耿玲,李辉. 灯盏乙素抗大鼠肝纤维化作用的研究[J]. 中国中药杂志,2015,40(10):1999-2003. [7] Zhang X,Ji R,Sun H,et al. Scutellarin ameliorates nonalcoholic fatty liver disease through the PPARgamma/PGC-1alpha-Nrf2 pathway[J]. Free Radic Res,2018,52(2):198-211. doi: 10.1080/10715762.2017.1422602 [8] Peng L,Wen L,Shi Q F,et al. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-kappaB/NLRP3-mediated epithelial-mesenchymal transition and inflammation[J]. Cell Death Dis,2020,11(11):978. doi: 10.1038/s41419-020-03178-2 [9] Su Y,Fan X,Li S,et al. Scutellarin improves type 2 diabetic cardiomyopathy by regulating cardiomyocyte autophagy and apoptosis[J]. Dis Markers,2022,2022(5):3058354. [10] Xue L J,Han J Q,Zhou Y C,et al. Untargeted metabolomics characteristics of nonobese nonalcoholic fatty liver disease induced by high-temperature-processed feed in Sprague-Dawley rats[J]. World J Gastroenterol,2020,26(46):7299-7311. doi: 10.3748/wjg.v26.i46.7299 [11] Videla L A,Pettinelli P. Misregulation of PPAR functioning and its pathogenic consequences associated with nonalcoholic fatty liver disease in human obesity[J]. PPAR Res,2012,2012(11):107434. [12] Yu H M,Chung H K,Park K S. The PDE5 inhibitor udenafil ameliorates nonalcoholic fatty liver disease by improving mitochondrial function[J]. Biochem Biophys Res Commun,2021,558(6):57-63. [13] 江静,李虎,彭宗根. 肝内巨噬细胞在肝纤维化发展中的双重作用及其靶向治疗研究进展[J]. 中国药学杂志,2021,56(23):1869-1873. [14] Kong D,Zhang Z,Chen L,et al. Curcumin blunts epithelial-mesenchymal transition of hepatocytes to alleviate hepatic fibrosis through regulating oxidative stress and autophagy[J]. Redox Biol,2020,36(5):101600. [15] Ren L,Qi K,Zhang L,et al. Glutathione might attenuate cadmium-induced liver oxidative stress and hepatic stellate cell activation[J]. Biol Trace Elem Res,2019,191(2):443-452. doi: 10.1007/s12011-019-1641-x [16] Dai W,Qin Q,Li Z,et al. Curdione and schisandrin c synergistically reverse hepatic fibrosis via modulating the tgf-beta pathway and inhibiting oxidative stress[J]. Front Cell Dev Biol,2021,9(11):763864. [17] 蔡菁,葛亚强,许丽娟,等. 蒿属香豆素对MCD饮食诱导的非酒精性脂肪性肝纤维化小鼠保护性作用的研究[J]. 陕西中医,2021,42(11):1511-1516. [18] Gao J,Chen G,He H,et al. Therapeutic effects of breviscapine in cardiovascular diseases:A review[J]. Front Pharmacol,2017,8(5):289. [19] Liu Q,Li X,Ouyang X,et al. Dual effect of glucuronidation of a pyrogallol-type phytophenol antioxidant:A comparison between scutellarein and scutellarin[J]. Molecules,2018,23(12):3225. doi: 10.3390/molecules23123225 [20] Algandaby M M,Al-Sawahli M M,Ahmed O,et al. Curcumin-zein nanospheres improve liver targeting and antifibrotic activity of curcumin in carbon tetrachloride-induced mice liver fibrosis[J]. J Biomed Nanotechnol,2016,12(9):1746-1757. doi: 10.1166/jbn.2016.2270 [21] Zheng W,Song Z,Li S,et al. Protective effects of sesamol against liver oxidative stress and inflammation in high-fat diet-induced hepatic steatosis[J]. Nutrients,2021,13(12):4484. doi: 10.3390/nu13124484 [22] He H,Xiong L,Jian L,et al. Role of mitochondria on UV-induced skin damage and molecular mechanisms of active chemical compounds targeting mitochondria[J]. J Photochem Photobiol B,2022,232(5):112464. [23] Mortezaee K. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and liver fibrosis:A review[J]. Cell Biochem Funct,2018,36(6):292-302. doi: 10.1002/cbf.3351 -





 下载:
下载: