Effect of Cathelicidin LL-37 on Transmembrane Barrier Function of Urothelial Cells
-
摘要:
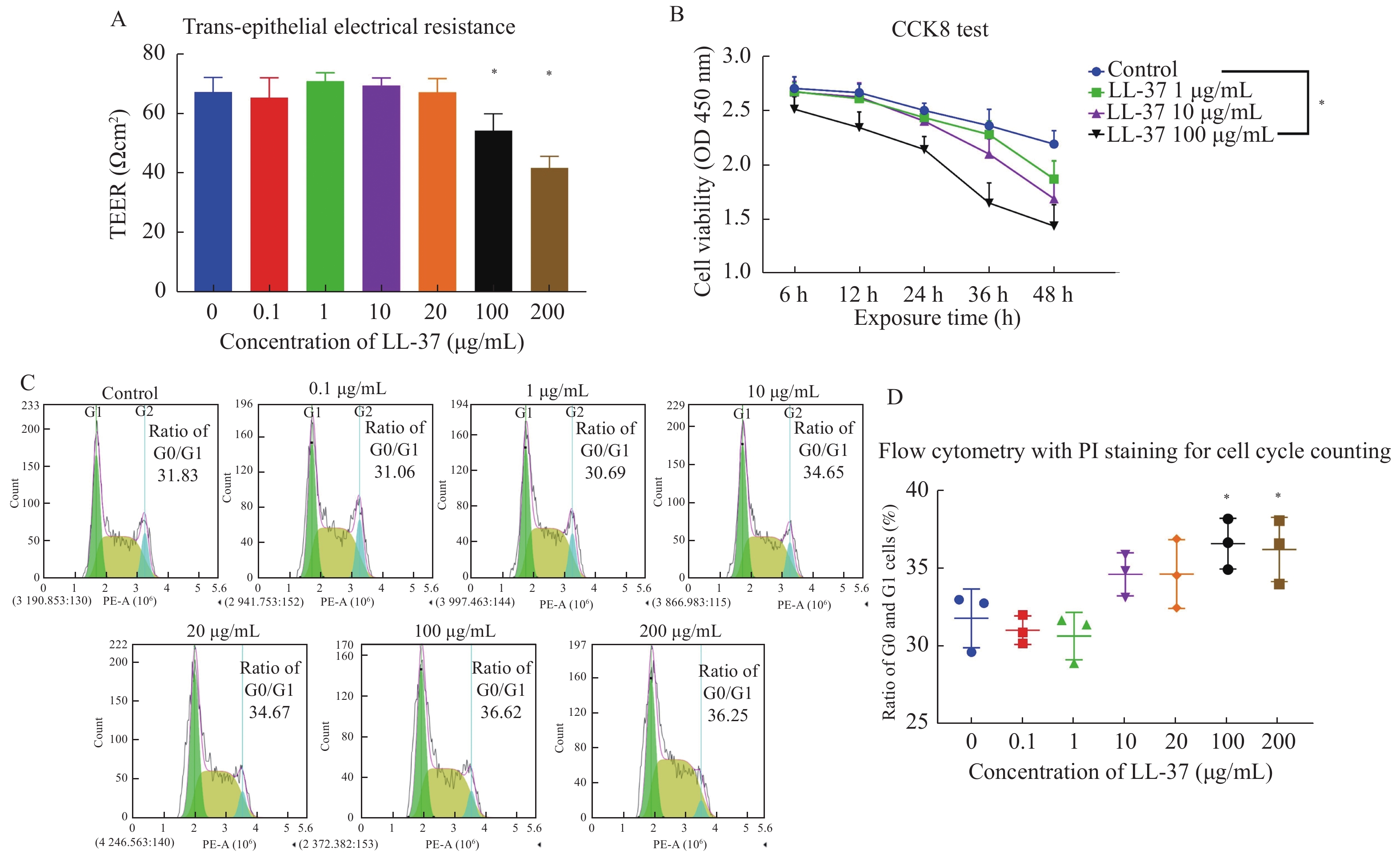
目的 探索LL-37对膀胱尿路上皮细胞跨膜屏障功能破坏的具体作用,构建适于体外研究间质性膀胱炎(interstitial cystitis,IC)的细胞实验模型。 方法 分别用不同浓度的LL-37处理人尿路上皮永生化细胞SV-HUC-1,通过跨上皮电阻测量仪测量跨上皮细胞电阻,CCK-8检测细胞增殖活力,流式细胞仪检测细胞周期和钙离子浓度,RT-qPCR和WB检测葡萄糖胺聚糖(GAGs)关键成分硫酸乙酰肝素的表达水平。 结果 体外细胞跨上皮电阻测量显示,100和200 μg/mL LL-37可显著抑制细胞的跨上皮电阻,破坏SV-HUC-1细胞的跨膜屏障功能。CCK-8和PI染色流式细胞检测显示,100 μg/mL 以上的LL-37可显著降低SV-HUC-1细胞的增殖活力,提高SV-HUC-1细胞处于G0/G1期的比例(P < 0.05),提示细胞增值受到显著抑制。进一步流式细胞术检测SV-HUC-1细胞中的钙离子浓度显示,LL-37处理后的尿路上皮细胞内钙离子浓度显著升高(P < 0.05),且增高量与LL-37浓度相关。RT-qPCR和WB检测证实经LL-37处理后SV-HUC-1细胞的GAGs关键成分硫酸乙酰肝素表达显著上调(P < 0.05)。 结论 抗菌肽LL-37可抑制SV-HUC-1细胞内GAGs关键成分硫酸乙酰肝素的表达,并破坏SV-HUC-1的细胞增殖和跨膜屏障功能。 Abstract:Objective To explore the specific effect of LL-37 on the destruction of the transmembrane barrier function of bladder urothelial cells and develop a cellular experimental model suitable for studying interstitial cystitis (IC) in vitro. Methods Human urothelial immortalized cells SV-HUC-1 were treated with LL-37 at different concentrations. The transepithelial electrical resistance (TEER) was measured by a transepithelial resistor, and the cell proliferative activity was detected by CCK-8 kit. Cell cycle and calcium ion concentration were detected by flow cytometry. The expression levels of heparan sulfate, a key component of glycosaminoglycan (GAGs), were detected by RT-qPCR and WB. Results In vitro measurement showed that 100 and 200 μg/mL of LL-37 significantly inhibited the TEER and destroyed the transmembrane barrier function of SV-HUC-1. The CCK-8 test and PI staining flow cytometry results showed that LL-37 above 100 μg/mL could significantly reduce the proliferation activity of SV-HUC-1 and increase the proportion of SV-HUC-1 in the G0/G1 phase (P < 0.05), suggesting that cell proliferation was significantly inhibited. Further flow cytometry analysis showed that the calcium ion concentration in SV-HUC-1treated with LL-37 was significantly increased (P < 0.05), and the increase was related to LL-37 concentration. RT-qPCR and WB assays confirmed significant up-regulation of heparan sulfate, a key GAGs component, in the LL-37-treated SV-HUC-1 (P < 0.05). Conclusion The cathelicidin LL-37 can inhibit the expression of GAGs and destroy the cell proliferation and transmembrane barrier function of SV-HUC-1. -
表 1 引物序列
Table 1. Primer sequences
目的基因 引物序列(F:正向序列;R:反向序列;5′-3′) HS2ST1 F: CAGCGGCGTTGGTGATAGCG R: AGAGAGCGACAGCGAGAGAACC HS3ST1 F: CCAAGTGTTCTACAACCACATG R: CTTTAGGAACCTCTCGACCTTT HS3ST2 F: CTTACCTGTGTTACAGCTTCCT R: CTTCTGGAGAAGTTTCTGGCC HS3ST3A1 F: AACCTGAACTCGGGCGGAGAG R: GTGGCGAGCGACAGTGACTTC HS6ST1 F: GAAGACGTCGTTGCATATGTG R: GATGAAGGACAGGTTGTAGCAG -
[1] Birder L A. Pathophysiology of interstitial cystitis[J]. International Journal of Urology, 2019, 26( Suppl 1): 12-15. [2] Marcu I,Campian E C,Tu F F. Interstitial cystitis/bladder pain syndrome[J]. Seminars in Reproductive Medicine,2018,36(2):123-135. doi: 10.1055/s-0038-1676089 [3] Daniels A M,Schulte A R,Herndon C M. Interstitial cystitis:An update on the disease process and treatment[J]. Journal of Pain & Palliative Care Pharmacotherapy,2018,32(1):49-58. [4] Theoharides T C, Kempuraj D, Sant G R. Mast cell involvement in interstitial cystitis: A review of human and experimental evidence[J]. Urology, 2001, 57(6 Suppl 1): 47-55. [5] Sahiner I F,Soylu H,Ates E,et al. Impact of intravesical hyaluronic acid treatment on bladder inflammation in interstitial cystitis rat model[J]. International Brazilian Journal of Urology,2018,44(5):1014-1022. doi: 10.1590/s1677-5538.ibju.2017.0713 [6] Jia W,Schults A J,Jensen M M,et al. Bladder pain in an LL-37 interstitial cystitis and painful bladder syndrome model[J]. American Journal of Clinical and Experimental Urology,2017,5(2):10-17. [7] Johansson J,Gudmundsson G H,Rottenberg M E,et al. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37[J]. The Journal of Biological Chemistry,1998,273(6):3718-3724. doi: 10.1074/jbc.273.6.3718 [8] Martin Jensen M,Jia W,Schults A J,et al. IL-33 mast cell axis is central in LL-37 induced bladder inflammation and pain in a murine interstitial cystitis model[J]. Cytokine,2018,110(30):420-427. [9] 王光,杨童欣,姜永明,等. 抗菌肽LL-37诱导大鼠膀胱壁肥大细胞的炎症反应[J]. 昆明医科大学学报,2019,40(6):5. [10] Nijnik A,Hancock R E. The roles of cathelicidin LL-37 in immune defences and novel clinical applications[J]. Current Opinion in Hematology,2009,16(1):41-47. doi: 10.1097/MOH.0b013e32831ac517 [11] Oottamasathien S, Jia W, McCoard L, et al. A murine model of inflammatory bladder disease: Cathelicidin peptide induced bladder inflammation and treatment with sulfated polysaccharides[J]. The Journal of Urology, 2011, 186(4 Suppl): 1684-1692. [12] Oottamasathien S, Jia W, Roundy L M, et al. Physiological relevance of LL-37 induced bladder inflammation and mast cells[J]. The Journal of Urology, 2013, 190(4 Suppl): 1596-1602. [13] Howard P S,Renfrow D,Schechter N M,et al. Mast cell chymase is a possible mediator of neurogenic bladder fibrosis[J]. Neurourology and Urodynamics,2004,23(4):374-382. doi: 10.1002/nau.20032 [14] Lee W Y,Savage J R,Zhang J,et al. Prevention of anti-microbial peptide LL-37-induced apoptosis and ATP release in the urinary bladder by a modified glycosaminoglycan[J]. PloS One,2013,8(10):77854. doi: 10.1371/journal.pone.0077854 [15] Song Y J,Cao J Y,Jin Z,et al. Inhibition of microRNA-132 attenuates inflammatory response and detrusor fibrosis in rats with interstitial cystitis via the JAK-STAT signaling pathway[J]. Journal of Cellular Biochemistry,2019,120(6):9147-9158. doi: 10.1002/jcb.28190 [16] Xie J,Liu B,Chen J,et al. Umbilical cord-derived mesenchymal stem cells alleviated inflammation and inhibited apoptosis in interstitial cystitis via AKT/mTOR signaling pathway[J]. Biochemical and Biophysical Research Communications,2018,495(1):546-552. doi: 10.1016/j.bbrc.2017.11.072 [17] Boudieu L,Mountadem S,Lashermes A,et al. Blocking α(2)δ-1 subunit reduces bladder hypersensitivity and inflammation in a cystitis mouse model by decreasing NF-kB pathway activation[J]. Frontiers in Pharmacology,2019,10(1):133. [18] Assi K,Pillai R,Gómez-Muñoz A,et al. The specific JNK inhibitor SP600125 targets tumour necrosis factor-alpha production and epithelial cell apoptosis in acute murine colitis[J]. Immunology,2006,118(1):112-121. doi: 10.1111/j.1365-2567.2006.02349.x [19] Henderson N C,Pollock K J,Frew J,et al. Critical role of c-jun (NH2) terminal kinase in paracetamol- induced acute liver failure[J]. Gut,2007,56(7):982-990. doi: 10.1136/gut.2006.104372 [20] Zhao J,Wang L,Dong X,et al. The c-Jun N-terminal kinase (JNK) pathway is activated in human interstitial cystitis (IC) and rat protamine sulfate induced cystitis[J]. Scientific Reports,2016,6(1):19670. doi: 10.1038/srep19670 -






 下载:
下载: