Application of CT Radiomics in the Short-Term Prognosis of Combined Therapy for Hepatocellular Carcinoma
-
摘要:
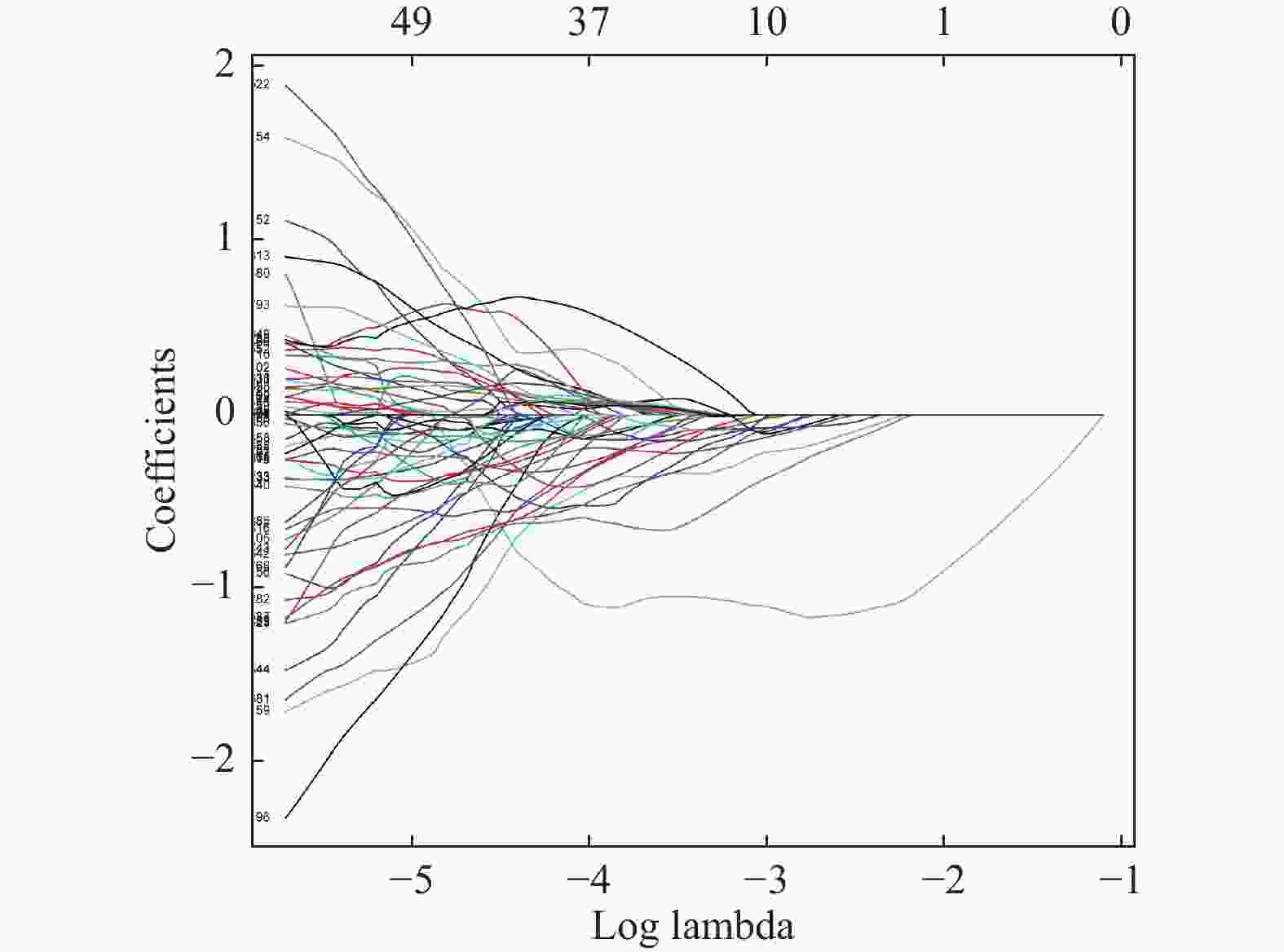
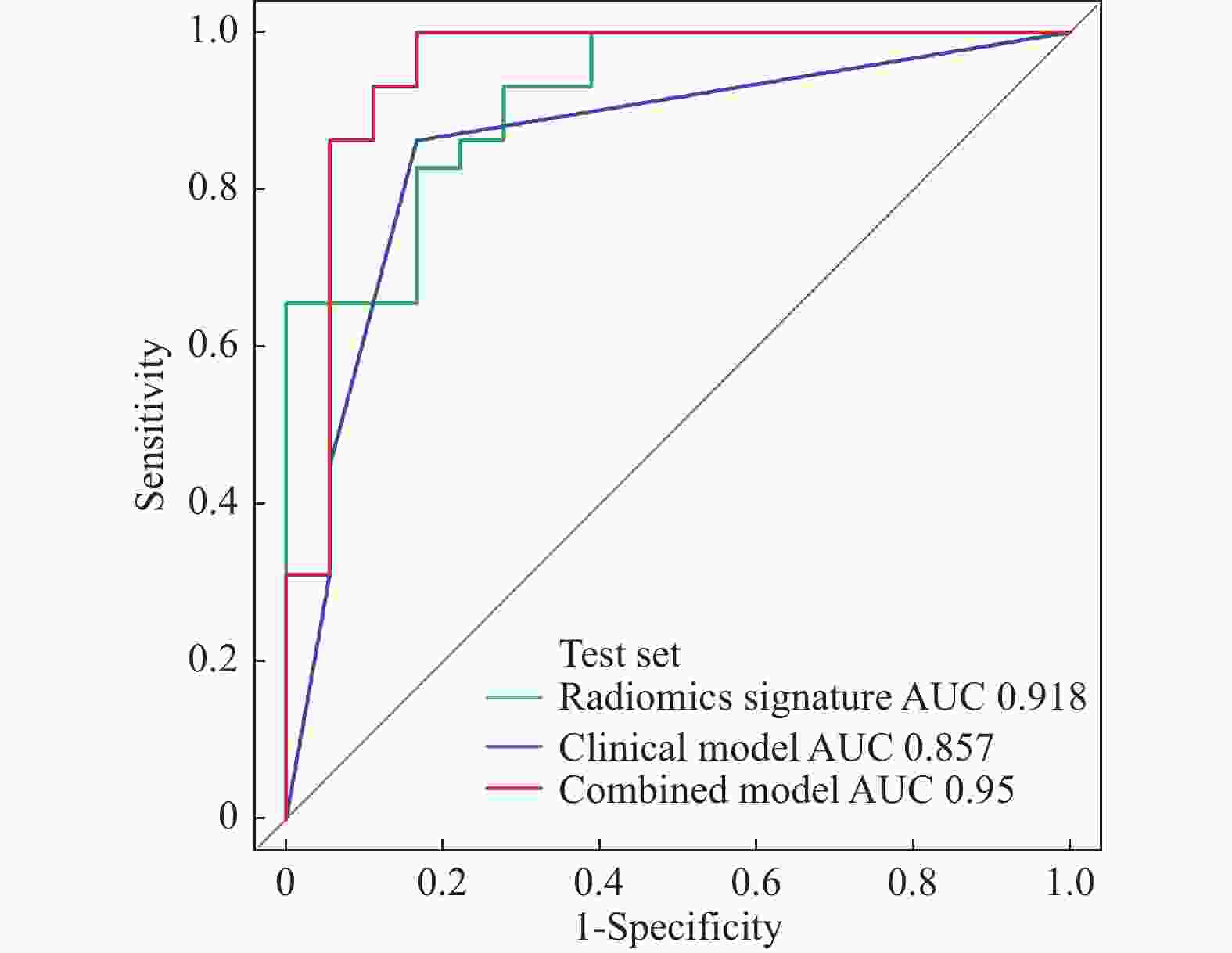
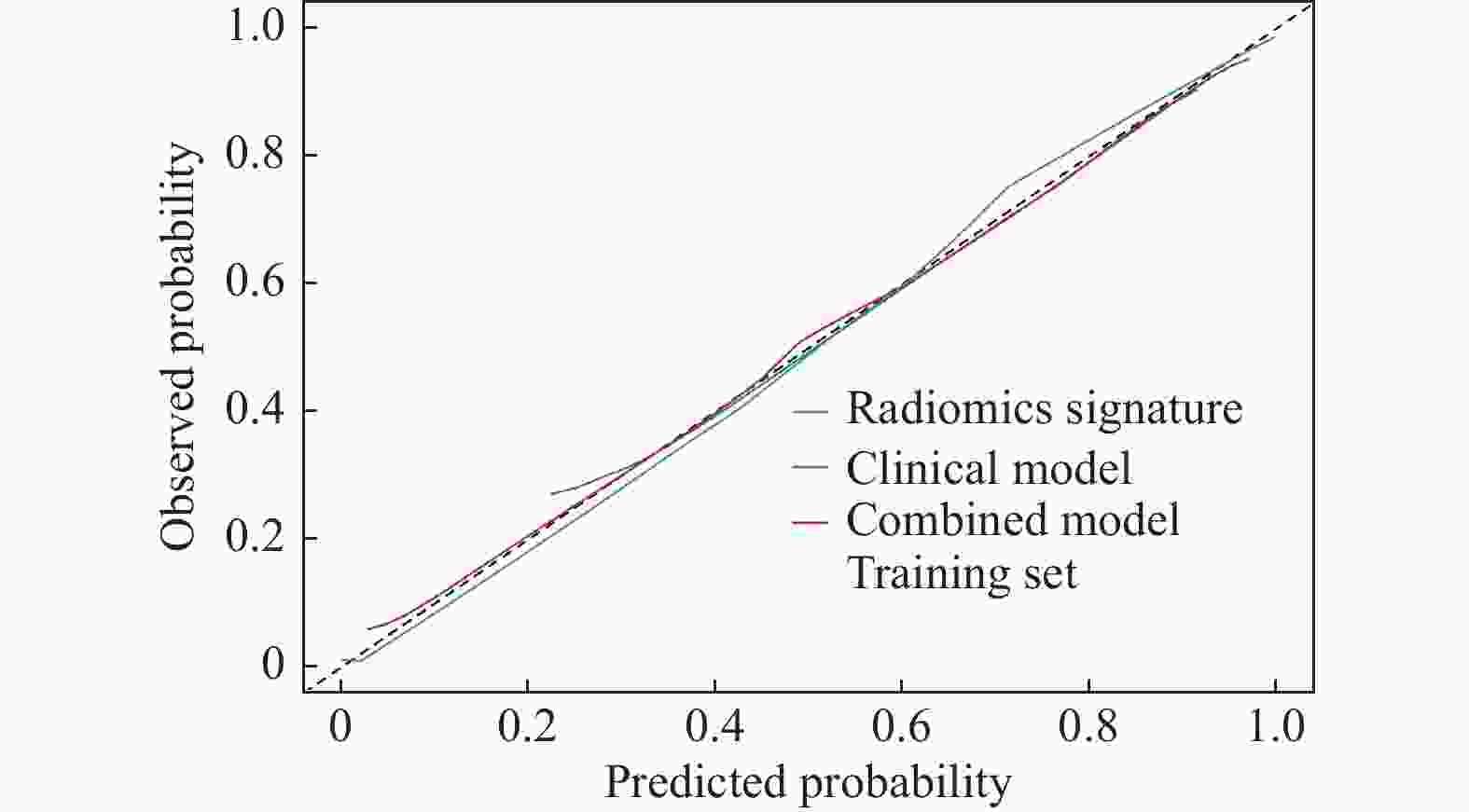
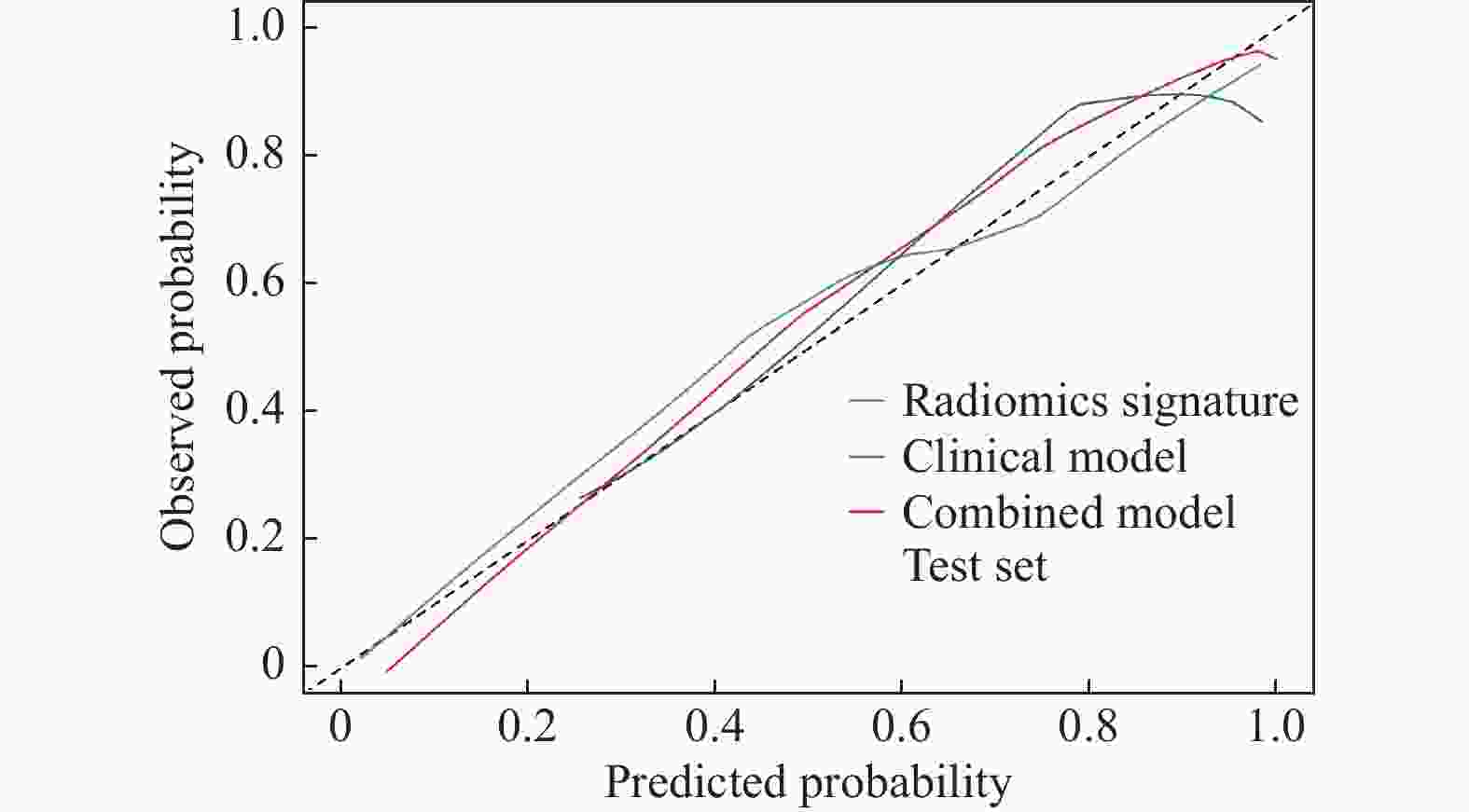
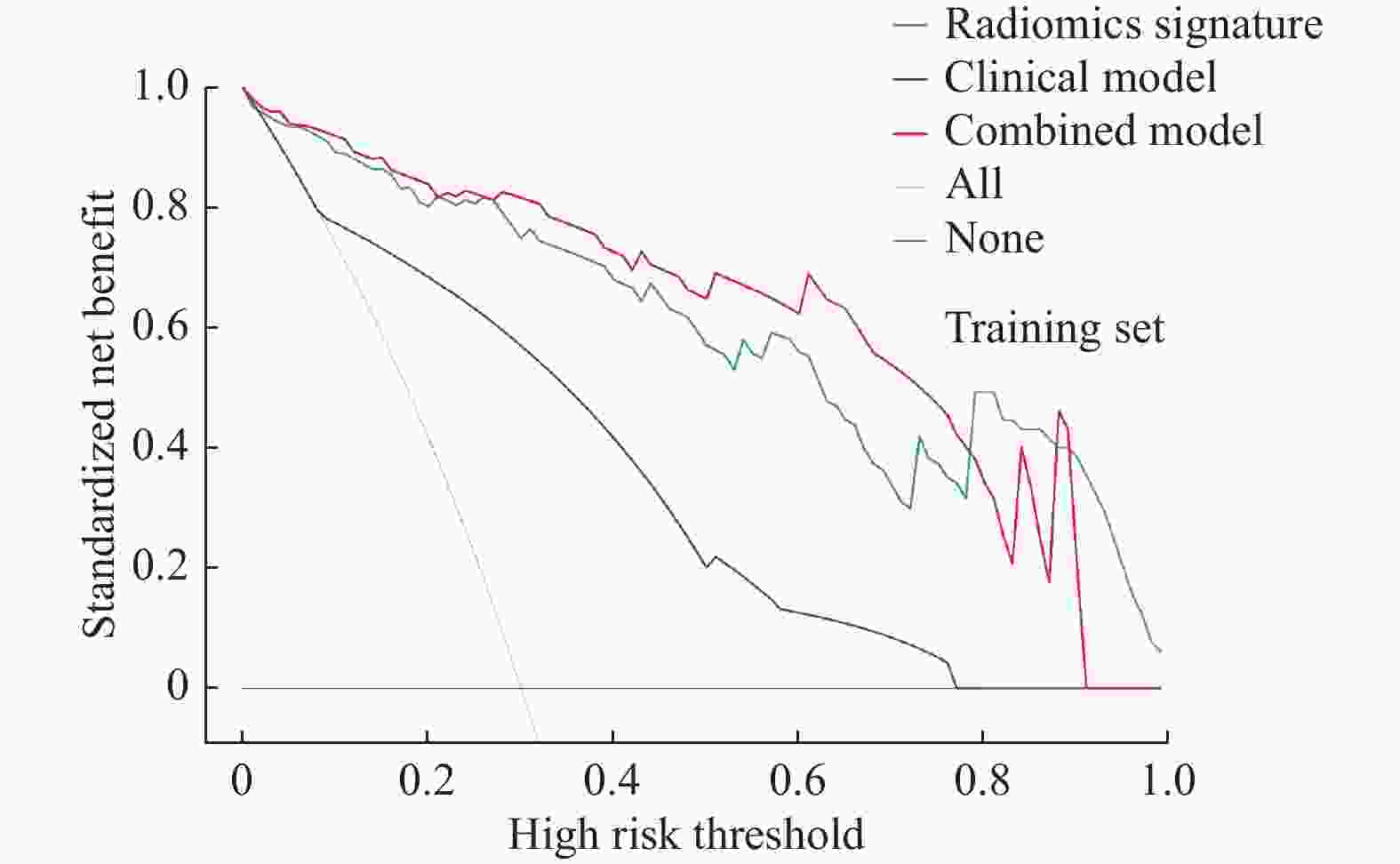
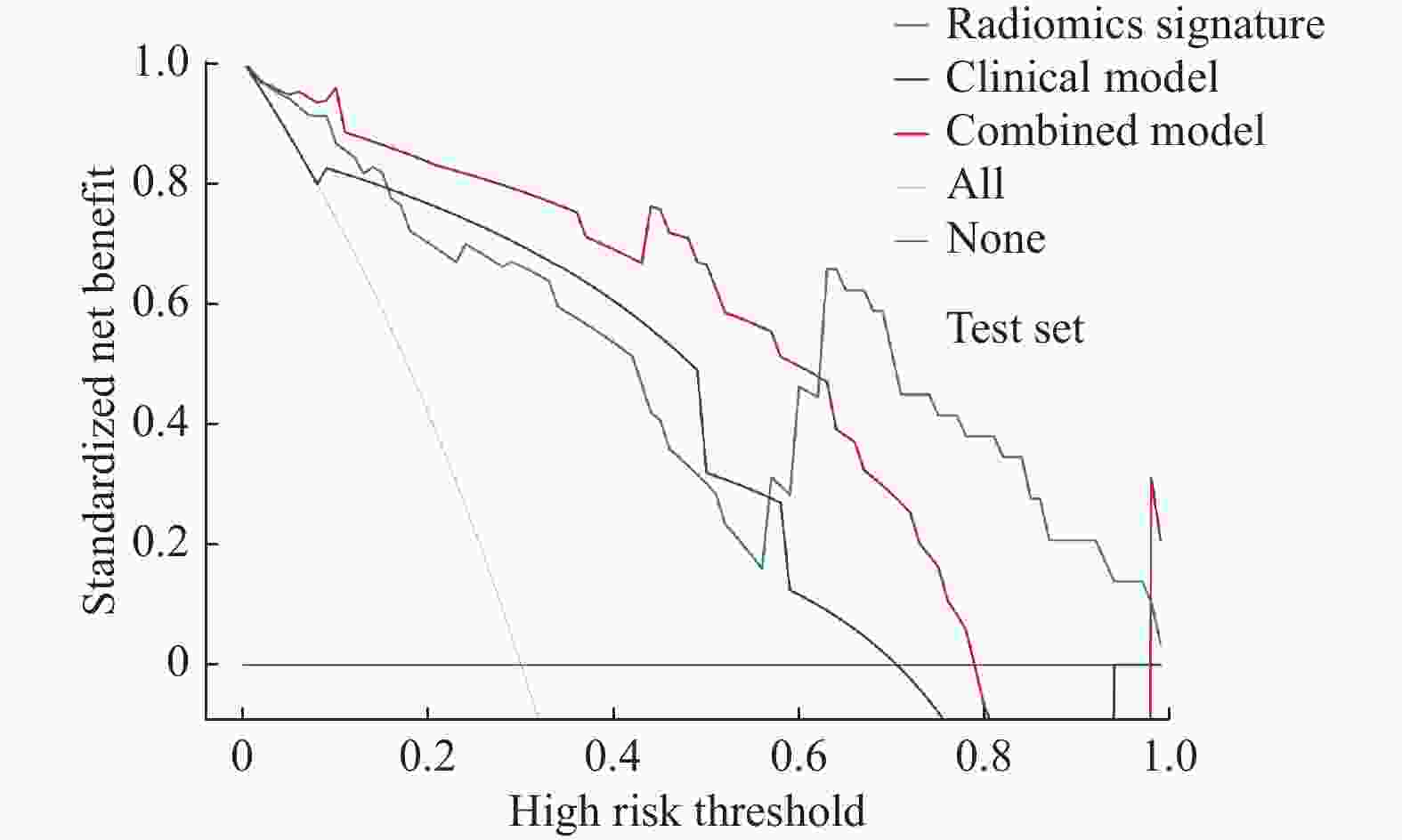
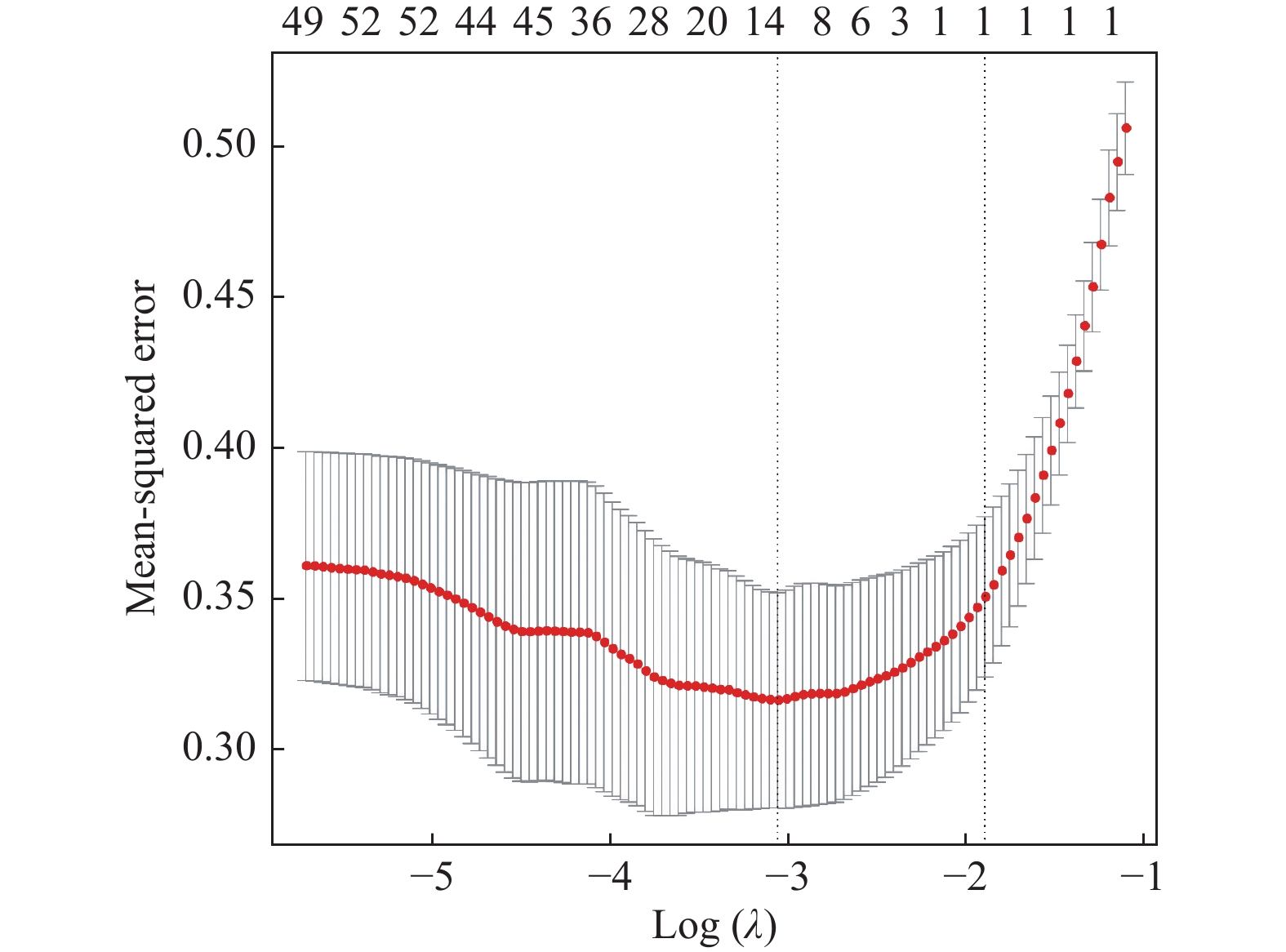
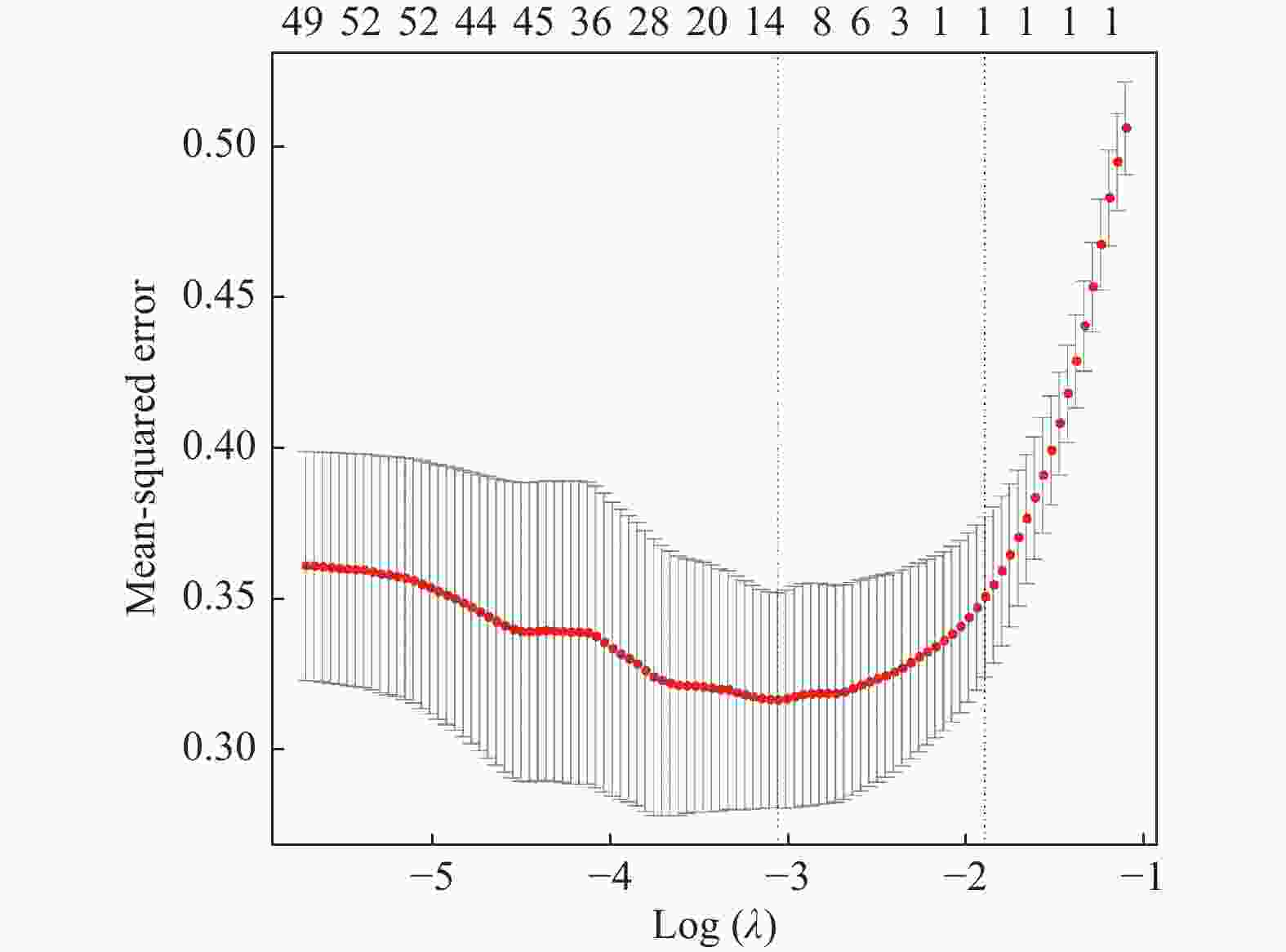
目的 探讨增强CT的影像组学模型在预测肝细胞癌(hepatocellular carcinoma,HCC)经导管肝动脉化疗栓塞(transcatheter arterial chemoembolization,TACE)联合索拉菲尼治疗后短期疗效(6个月内)中的价值。 方法 回顾性分析159例TACE联合索拉菲尼治疗的肝癌患者的临床、病理及术前CT图像,根据改良后实体瘤疗效评价标准(modified response evaluation criteria in solid tumors,mRECIST)对介入术后 6 个月肿瘤局部控制率进行评估。提取动脉期和门脉期的影像组学特征并构建影像组学模型。根据术前一般临床危险因素、基本影像特征,建立术前临床模型。将影像组学模型加入术前临床模型,组成联合模型。分别绘制3个模型的受试者工作特征曲线(ROC),并分别计算曲线下面积(AUC)。 结果 从1 708个影像组学特征中共筛选出11个影像组学特征值建立影像组学模型,训练组及验证组模型AUC分为0.945、0.918。临床因素除了肿瘤最大径线和肿瘤数目外,差异有统计学意义(P < 0.05),其余差异无统计学意义(P > 0.05),二者构建的临床模型训练组及验证组的AUC分别为0.811、0.857。联合模型训练组及验证组AUC分别为0.958、0.95。 结论 影像组学模型是预测TACE联合索拉菲尼短期疗效的一个强有力的独立预测因素,能明显提高术前临床模型的预测能力,可在TACE联合索拉菲尼治疗前筛选出进展危险性较高的患者,帮助临床医生制定个性化的治疗及随访方案,改善患者预后。 Abstract:Objective To investigate the value of enhanced CT radiomics model in predicting the short-term efficacy (within 6 months) of TACE combined with sorafenib in hepatocellular carcinoma. Method The clinical, pathological and preoperative CT images of 159 HCC patients treated with TACE combined with solafenib were retrospectively analyzed. Local tumor control rate was assessed according to the modified Response Evalaution Criteria in Solid Tumors (mRECIST) 6 months later. The imaging features of arterial and portal phases were extracted to establish radiomics model. The general preoperative clinical risk factors and basic imaging features were analyzed to establish a preoperative clinical model. Radiomics model were added to the preoperative clinical model to form a combined model. The receiver operating characteristic curves (ROC) of the three models were drawn and calculate the area under the curve (AUC) respectively. Results A total of 11 feature values were selected from 1 708 radiomic features to establish the raduomics model. The AUC of training group and the validation group was 0.945 and 0.918 respectively. Except the maximum diameter line and number of tumors, the differences were statistically significant (P < 0.05), while the other differences were not statistically significant (P > 0.05). The AUC of the clinical model training group and the validation group was 0.811 and 0.857 respectively. The AUC of the combined model training group is 0.958, and that of validation group is about 0.95. Conclusion The radiomics model constructed in this study is a powerful independent predictor for predicting the short-term efficacy of TACE combined with sorafenib, which can significantly improve the predictive ability of preoperative clinical model, screen patients with higher risk of progression before TACE combined with sorafenib treatment, and help clinicians to develop personalized treatment and follow-up plan to improve the prognosis of patients. -
表 1 训练集和验证集的临床参数比较[n(%)]
Table 1. Comparison of clinical parameters between the training group and the validation group [n(%)]
变量 训练队列
(n = 112)验证队列
(n = 47)χ2 P 年龄(岁) 0.100 0.752 < 60 65(58) 26(55.3) ≥60 47(42) 21(44.7) 性别 4.309 0.038* 男 100(89.3) 36(76.6) 女 12(10.7) 11(23.4) 肿瘤最大径(cm) 0.085 0.770 < 5 36(32.1) 14(29.8) ≥5 76(67.9) 33(70.2) 肿瘤数目 1.341 0.247 单发 46(41) 24(51) 多发 66(58.9) 23(49) 肝炎病史 6.872 0.009* 是 75(67) 21(44.7) 否 37(33) 26(55.3) 肝硬化 0.303 0.582 是 59(52.7) 27(57.4) 否 53(47.3) 20(42.6) 肝包膜 1.004 0.316 是 16(14.3) 4(8.5) 否 96(85.7) 43(91.5) *P < 0.05。 表 2 临床参数的单因素和多因素逻辑回归分析
Table 2. Univariate and multivariate logistic regression analysis of clinical parameters
临床因素 单因素Logistic 回归分析 多因素Logistic 回归分析 B P OR (95% CI) B P OR (95% CI) 年龄 0.414 0.292 1.513(0.701~3.265) 肿瘤最大径 −2.036 < 0.001* 0.131(0.046~0.372) −2.484 < 0.001* 0.083(0.026~0.265) 性别 −0.857 0.218 0.424(0.108~1.661) 肿瘤数目 −1.719 < 0.001* 0.179(0.075~0.431) −2.153 < 0.001* 0.116(0.043~0.314) 肝包膜 1.299 0.053 3.667(0.981~13.699) 肝硬化 0.112 0.771 1.118(0.527~2.371) 肝炎病史 0.243 0.549 1.275(0.576~2.822) 常量 3.488 32.709 *P < 0.05。 -
[1] Chen R X,Gan Y H,Ge N L,et al. A new prediction model for prognosis of patients with intermediate-stage HCC after conventional transarterial chemoembolization:An internally validated study[J]. J Cancer,2019,10(26):6535-6542. doi: 10.7150/jca.34064 [2] Qiu Z,Shen L,Jiang Y,et al. Transarterial chemoembolization (tace) combined with apatinib versus tace combined with sorafenib in advanced hepatocellular carcinoma patients:A multicenter retrospective study[J]. Ann Transl Med,2021,9(4):283-296. doi: 10.21037/atm-20-5360 [3] 李群,王燕,周文辉,等. 经动脉化疗栓塞联合微波消融治疗多结节肝细胞癌的短期疗效分析[J]. 实用放射学杂志,2019,35(4):626-629. doi: 10.3969/j.issn.1002-1671.2019.04.028 [4] Koroki K,Ogasawara S,Ooka Y,et al. Analyses of intermediate-stage hepatocellular carcinoma patients receiving transarterial chemoembolization prior to designing clinical trials[J]. Liver Cancer,2020,9(5):596-612. doi: 10.1159/000508809 [5] Hyoung,Ook,Kim,et al. Change in perfusion angiography during transcatheter arterial chemoembolization for hepatocellular carcinoma predicts short-term outcomes[J]. AJR Am J Roentgenol,2019,213(4):746-754. doi: 10.2214/AJR.18.20499 [6] Stampfl U,Bermejo J L,Sommer C M,et al. Efficacy and nontarget effects of transarterial chemoembolization in bridging of hepatocellular carcinoma patients to liver transplantation:A histopathologic study[J]. J Vasc Interv Radiol,2014,25(7):1018-1026. doi: 10.1016/j.jvir.2014.03.007 [7] Zou X,Fan W,Xue M,et al. Evaluation of the benefits of tace combined with sorafenib for hepatocellular carcinoma based on untreatable tace (untaceable) progression[J]. Cancer Manag Res,2021,52(6):4013-4029. [8] Kimura Y,Kaneko R,Yano Y,et al. The prognosis of hepatocellular carcinoma treated with sorafenib in combination with tace[J]. Asian Pac J Cancer Prev,2020,21(6):1797-1805. doi: 10.31557/APJCP.2020.21.6.1797 [9] 陈培培,张涛,张学琴,等. 影像学预测肝细胞癌微血管侵犯的研究进展[J]. 实用放射学杂志,2019,35(11):1865-1868. doi: 10.3969/j.issn.1002-1671.2019.11.038 [10] 王苏丹,张琦,刘明明,等. 精准医疗时代放射基因组学在肝细胞癌早期诊断中的应用[J]. 实用放射学杂志,2018,34(5):789-791. doi: 10.3969/j.issn.1002-1671.2018.05.038 [11] Heimbach J K,Kulik L M,Finn R S,et al. AASLD guidelines for the treatment of hepatocellular carcinoma[J]. Hepatology,2018,67(1):358-380. doi: 10.1002/hep.29086 [12] Lencioni R,Llovet J M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma[J]. Semin Liver Dis,2010,30(1):52-60. doi: 10.1055/s-0030-1247132 [13] Sun Y,Bai H,Xia W,et al. Predicting the outcome of transcatheter arterial embolization therapy for unresectable hepatocellular carcinoma based on radiomics of preoperative multiparameter MRI[J]. J Magn Reson Imaging,2020,52(4):1083-1090. doi: 10.1002/jmri.27143 [14] Jia F,Wu B,Yan R,et al. Prediction model for intermediate-stage hepatocellular carcinoma response to transarterial chemoembolization[J]. J Magn Reson Imaging,2020,52(6):1657-1667. doi: 10.1002/jmri.27189 [15] Fiz F,Viganò L,Gennaro N,et al. Radiomics of liver metastases:A systematic review[J]. Cancers (Basel),2020,12(10):2881-2904. doi: 10.3390/cancers12102881 [16] Ni J Y,Fang Z T,Sun H L,et al. A nomogram to predict survival of patients with intermediate-stage hepatocellular carcinoma after transarterial chemoembolization combined with microwave ablation[J]. Eur Radiol,2020,30(4):2377-2390. doi: 10.1007/s00330-019-06438-8 [17] Cc A,Hq B,Yy C,et al. Comprehensive predictive factors for calliSpheres microspheres (CSM) drug-eluting bead-transarterial chemoembolization and conventional transarterial chemoembolization on treatment response and survival in hepatocellular carcinoma patients - sscience direct[J]. Clin Res in Hepatol Gastroenterol,2020,45(2):1460-1470. [18] Philips C A,Rajesh S,Nair,D C,et al. Hepatocellular carcinoma in 2021:An exhaustive update[J]. Cureus,2021,13(11):19274-19293. -





 下载:
下载: