Effects of Dose-response and Time-dependent of Endotoxin on Proliferation of Human Umbilical Cord Mesenchymal Stem Cells
-
摘要:
目的 探讨内毒素(Endotoxin),又称为脂多糖(lipopolysaccharide,LPS)的量效和时效对人脐带间充质干细胞(human umbilical cord mesenchymal stem cells,hUC-MSCs)增殖的影响。 方法 用含不同浓度LPS(0.098、0.195、0.39、0.78、1.56、3.12、6.25、12.5、25和50 EU/mL)的干细胞培养基培养hUC-MSCs,采用CCK-8增殖实验检测24h、48h和72 h的细胞增殖情况。确定促进hUC-MSCs增殖的低浓度(0.098 EU/mL)、中浓度(1.56 EU/mL)和高浓度(25 EU/mL)LPS,选择作用时间72 h,进一步用流式细胞术明确LPS对hUC-MSCs增殖的影响。 结果 CCK-8检测结果提示,作用24 h、48 h时,表现为抑制hUC-MSCs的增殖。作用72 h时,表现为促进细胞增殖,且低浓度区域促进作用最显著,浓度增加其促增殖作用相对减弱。流式细胞术结果提示低浓度LPS组G0/G1期的占比降低,S期和G2/M期占比增高。 结论 LPS的量效和时效对hUC-MSCs的增殖表现出不同的效应,适宜的LPS浓度及作用时间有利于hUC-MSCs的增殖。 Abstract:Objective To investigate the effects of dose-response and time-dependent of endotoxin on the proliferation of human umbilical cord mesenchymal stem cells(hUC-MSCs). Methods The hUC-MSCs were cultured in stem cell culture medium containing different concentrations of endotoxin(0.098, 0.195, 0.390, 0.780, 1.5600, 3.12, 6.250, 12.500, 25.000 and 50.000 EU/mL). CCK-8 proliferation assay was used to detect the cell proliferation at 24, 48 and 72 hours. The low concentration(0.098 EU/mL), medium concentration(1.56 EU/mL)and high concentration(25 EU/mL) of LPS were determined to promote the proliferation of hUC-MSCs, and the action time was selected for 72 hours. The effect of LPS on the proliferation of hUC-MSCs was further determined by flow cytometry. Results After 24, 48 hours of the treatment, it was mainly manifested by inhibiting the proliferation of hUC-MSCs. After 72 hours of the treatment, it showed a trend of promoting cell proliferation, and the promotion effect was most significant in the low concentration area, and the promotion effect was relatively weakened when the concentration increased. Further flow cytometry results suggested that the proportion of G0/G1 phase decreased, S phase and G2/M phase increased in LPS group of low concentration. Conclusion The dose-response and time-dependent of endotoxin have different effects on the proliferation of hUC-MSCs , and the appropriate concentration and duration of action of endotoxin are beneficial to the proliferation of hUC-MSCs. -
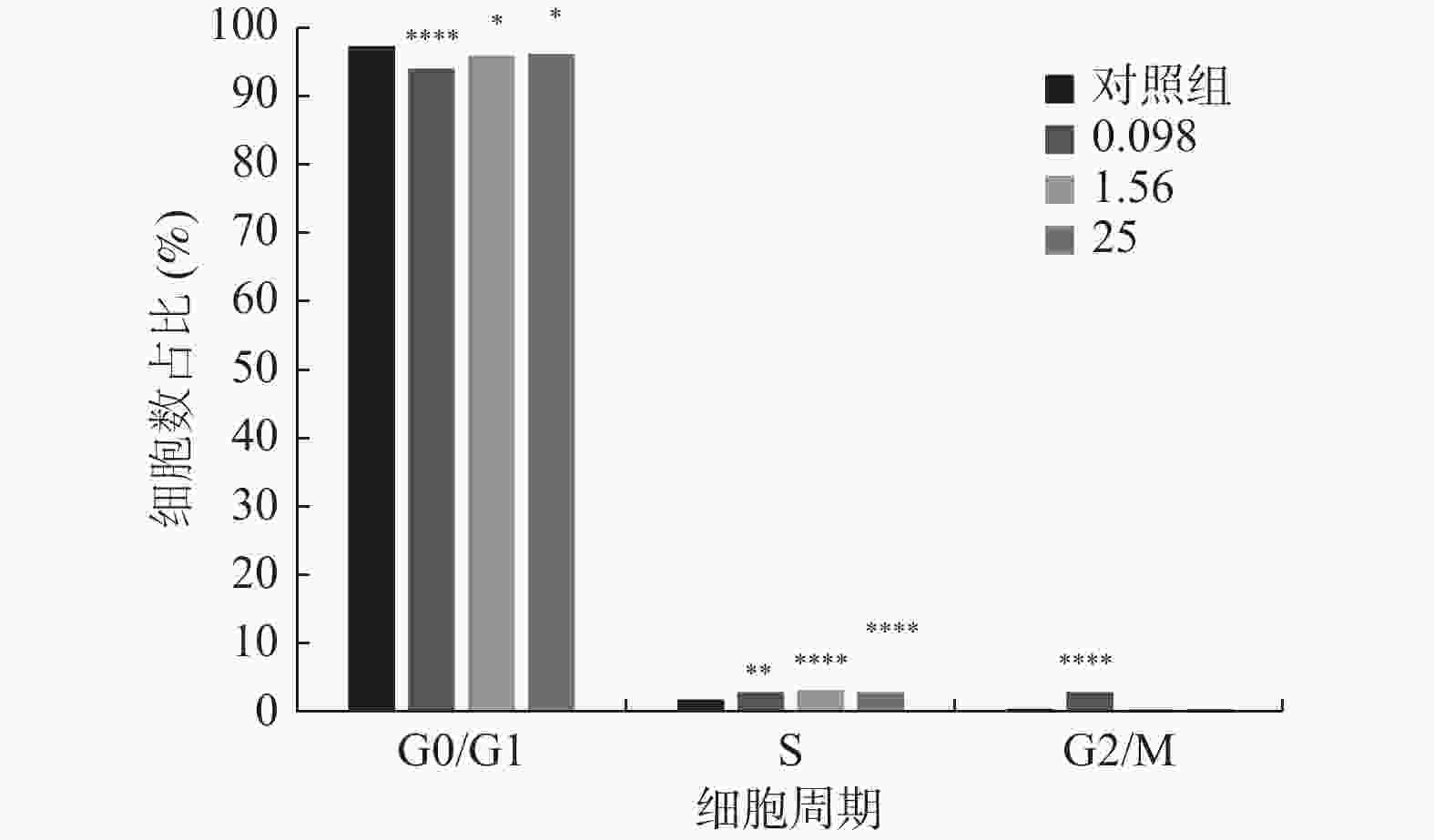
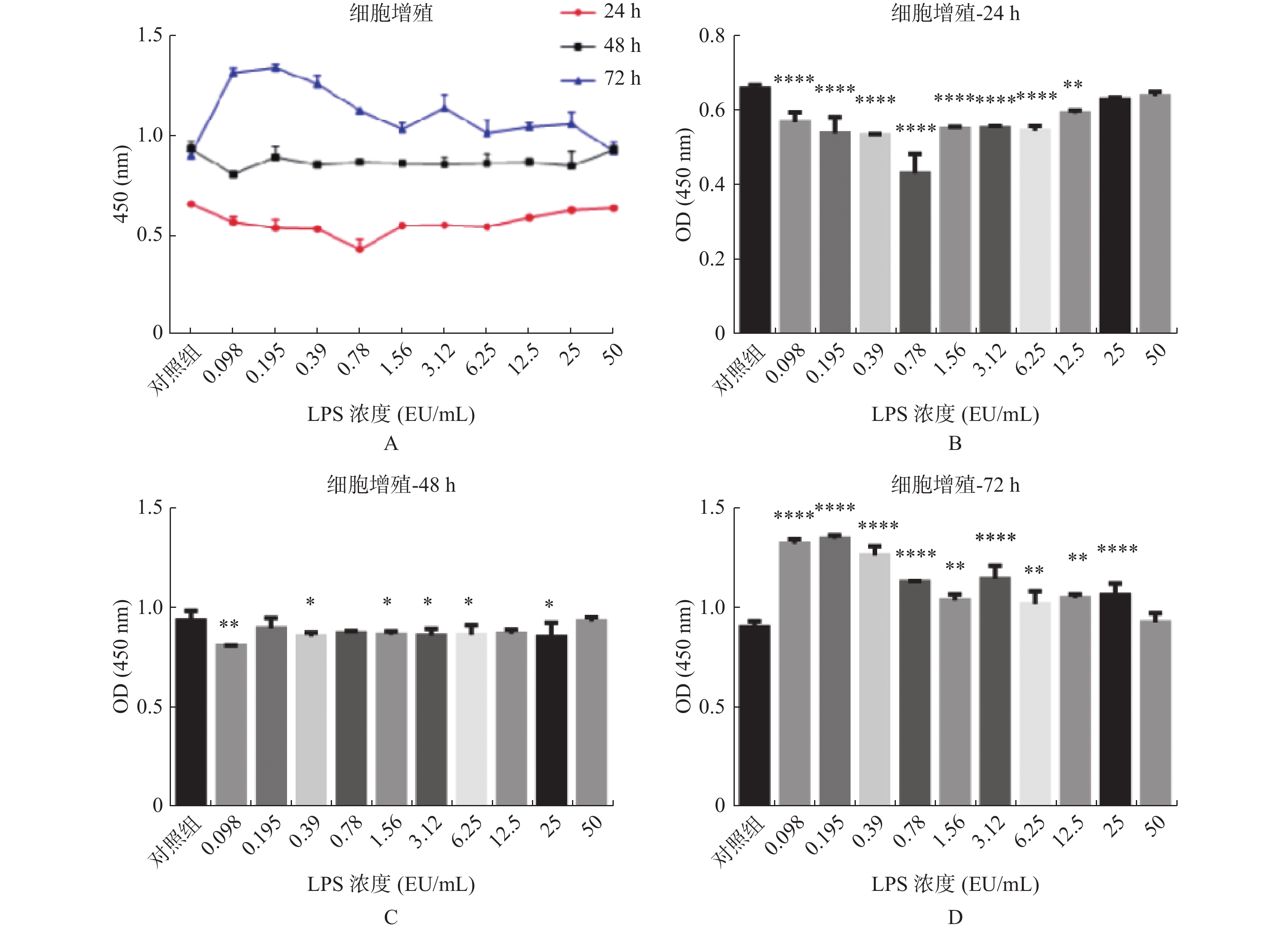
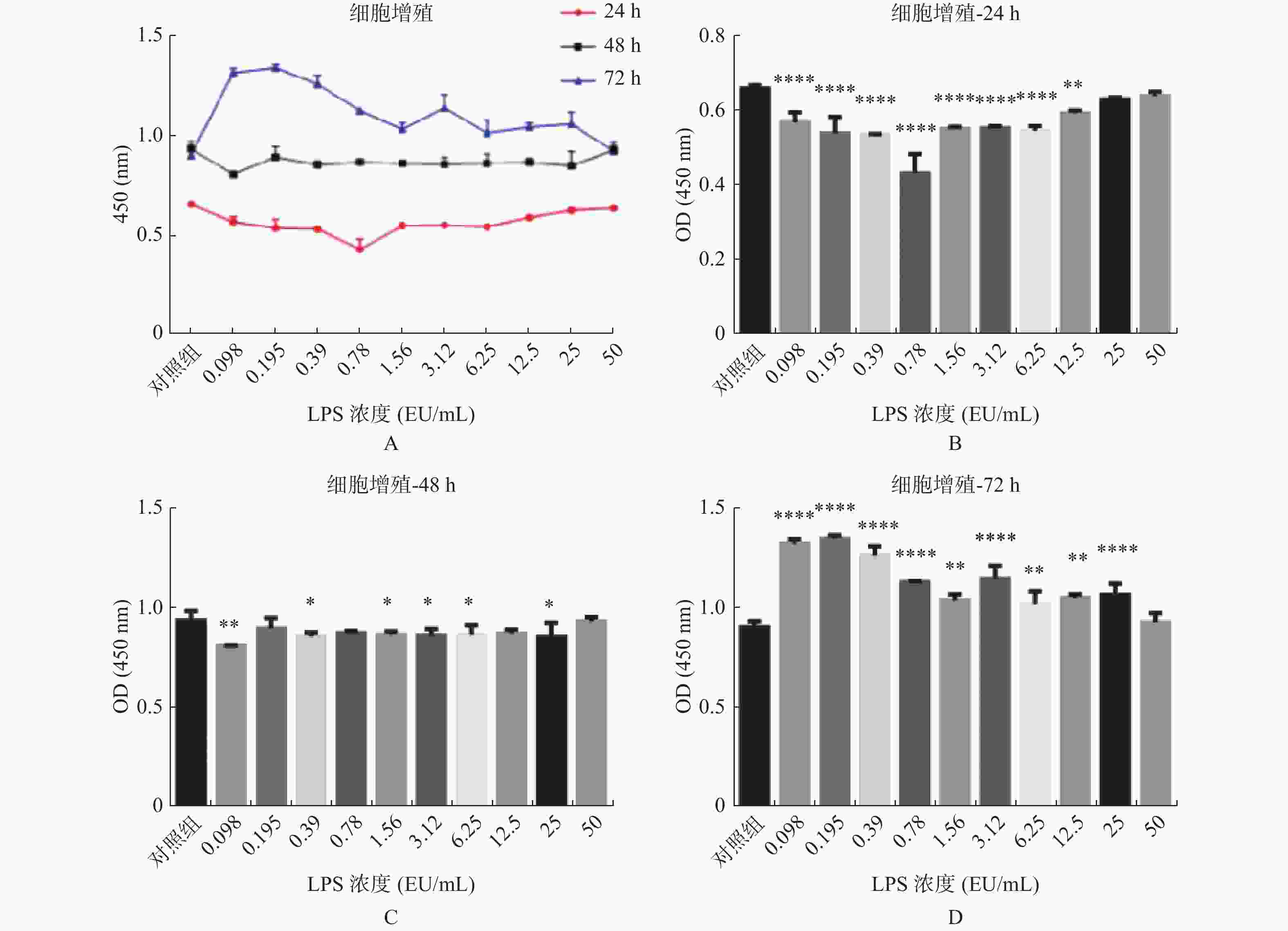
图 1 不同浓度及不同作用时间的内毒素对hUC-MSCs增殖的影响
A:LPS不同浓度及不同作用时间hUC-MSCs增殖情况;B:不同浓度LPS作用24 h hUC-MSCs增殖情况;C:不同浓度LPS作用48 h hUC-MSCs增殖情况;D:不同浓度LPS作用72 h hUC-MSCs增殖情况。与对照组比较,*P < 0.05,**P < 0.01,***P < 0.001,****P < 0.0001。
Figure 1. Effects of endotoxin with different concentrations and different time on the proliferation of hUC-MSCs
表 1 LPS不同浓度及不同作用时间hUC-MSCs吸光度(A)值(
$ \bar x \pm s $ )Table 1. Absorbance (A) of hUC-MSCs at different dose-response and time-dependent of LPS (
$ \bar x \pm s $ )组别 hUC-MSCs增殖 24 h 48 h 72 h 实验组1 0.571 ± 0.015* 0.811 ± 0.001* 1.321 ± 0.013* 实验组2 0.540 ± 0.025* 0.897 ± 0.030 1.346 ± 0.009* 实验组3 0.535 ± 0.002* 0.858 ± 0.011* 1.263 ± 0.026* 实验组4 0.433 ± 0.030* 0.871 ± 0.008 1.129 ± 0.003* 实验组5 0.552 ± 0.003* 0.865 ± 0.010* 1.037 ± 0.018* 实验组6 0.554 ± 0.003* 0.861 ± 0.020* 1.145 ± 0.038* 实验组7 0.547 ± 0.008* 0.865 ± 0.028* 1.018 ± 0.038* 实验组8 0.593 ± 0.005* 0.870 ± 0.012 1.049 ± 0.011* 实验组9 0.630 ± 0.003 0.853 ± 0.041* 1.063 ± 0.034* 实验组10 0.639 ± 0.007 0.933 ± 0.012 0.927 ± 0.027 对照组 0.660 ± 0.004 0.940 ± 0.020 0.906 ± 0.012 注:实验组1至10LPS终浓度依次为:0.098、0.195 、0.39 、0.78 、1.56 、3.12 、6.25 、12.5、25、50 EU/mL,表示分别在24 h、48 h、72 h时,与对照组相比,*P < 0.05。 表 2 LPS不同浓度及不同作用时间hUC-MSCs增殖率(%)
Table 2. Proliferation rates of hUC-MSCs at different dose-response and time-dependent of LPS (%)
时间 组1 组2 组3 组4 组5 组6 组7 组8 组9 组10 对照组 24 h 86.46 81.82 81.11 65.61 83.69 83.99 82.88 89.8 95.45 96.82 100.05 48 h 86.24 95.43 91.31 92.7 91.99 91.56 92.06 92.55 90.74 99.26 100 72 h 145.81 148.53 139.37 124.61 114.5 126.34 112.36 115.75 117.29 102.32 100 注:实验组1至10LPS终浓度依次为:0.098、0.195 、0.39 、0.78 、1.56 、3.12 、6.25 、12.5、25、50 EU/mL。 表 3 LPS对hUC-MSCs细胞周期的影响(%)
Table 3. Effect of LPS on huc-Mscs cell cycle (%)
分期 实验组1 实验组2 实验组3 对照组 G0/G1期 94.240 ± 0.450* 96.210 ± 0.323* 96.330 ± 0.254* 97.550 ± 0.300 S期 2.880 ± 0.144* 3.310 ± 0.144* 3.060 ± 0.121* 1.900 ± 0.110 G2/M期 2.880 ± 0.248* 0.480 ± 0.064 0.610 ± 0.075 0.550 ± 0.075 注:实验组1、2、3的LPS终浓度分别为0.098 EU/mL、1.56 EU/mL、25 EU/mL,对照组不含LPS;与对照组相比,*P < 0.05。 -
[1] Lou S,Duan Y,Nie H,et al. Mesenchymal stem cells:Biological characteristics and application in disease therapy[J]. Biochimie,2021,185(3):9-21. [2] Yi X,Liu F,Chen F,et al. Comparison of biological characteristics of placenta mesenchymal stem cells derived from fetus[J]. Chinese Journal of Biotechnology,2022,38(3):1183-1196. [3] Qin Z,Hua S,Chen H,et al. Parathyroid hormone promotes the osteogenesis of lipopolysaccharide-induced human bone marrow mesenchymal stem cells through the JNK MAPK pathway[J]. Bioscience Reports,2021,41(8):BSR20210420. doi: 10.1042/BSR20210420 [4] Qi T,Weng J,Yu F,et al. Insights into the role of magnesium ions in affecting osteogenic differentiation of mesenchymal stem cells[J]. Biological Trace Element Research,2021,199(2):559-567. doi: 10.1007/s12011-020-02183-y [5] Bagge J,Macleod J N,Berg L C. Cellular proliferation of equine bone marrow and adipose tissue-derived mesenchymal stem cells decline with increasing donor age[J]. Frontiers in Veterinary Science,2020,7(10):602403. [6] 高方媛,王宪波. 肠源性内毒素血症在肝衰竭发生发展中的作用[J]. 临床肝胆病杂志,2014,30(8):825-828. [7] Wang X,Ni J,You Y,et al. SNX10-mediated LPS sensing causes intestinal barrier dysfunction via a caspase-5-dependent signaling cascade[J]. The EMBO Journal,2021,40(24):e108080. [8] 丁晨. 脂多糖对人骨髓间充质干细胞增殖和成骨分化能力的影响及其相关机制的研究[D]. 上海: 第二军医大学硕士学位论文, 2017. [9] Camilleri M,Madsen K,Spiller R,et al. Intestinal barrier function in health and gastrointestinal disease:Intestinal barrier function[J]. Neurogastroenterology & Motility,2012,24(6):503-512. [10] Neves A L,Coelho J,Couto L,et al. Metabolic endotoxemia:A molecular link between obesity and cardiovascular risk[J]. Journal of Molecular Endocrinology,2013,51(2):51-64. doi: 10.1530/JME-13-0079 [11] Mulak A,Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease[J]. World Journal of Gastroenterology,2015,21(37):10609-10620. doi: 10.3748/wjg.v21.i37.10609 [12] 侯玉森,柴家科,刘玲英,等. 脂多糖预处理对人脐带间充质干细胞内毒素耐受的影响[J]. 中华医学杂志,2014,94(12):948-951. doi: 10.3760/cma.j.issn.0376-2491.2014.12.018 [13] 梁力建,李绍强. 缺血预处理对肝硬化肝癌患者入肝血流阻断肝切除的保护作用及其机理探讨[J]. 中华外科杂志,2002,40(4):28-30. [14] 朱路文,叶涛,姜云飞,等. 电针预处理对大鼠脑缺血再灌注损伤后炎性因子及细胞凋亡的影响[J]. 中国康复理论与实践,2016,22(7):765-768. doi: 10.3969/j.issn.1006-9771.2016.07.005 [15] 陈裕洁,龚楚链,谭芳,等. 右美托咪啶预处理对脓毒症肾损伤大鼠炎性因子和氧化应激的影响[J]. 南方医科大学学报,2015,35(10):1472-1475,1491. doi: 10.3969/j.issn.1673-4254.2015.10.20 [16] 李晓泓,张露芬,解秸萍,等. 艾灸预处理对佐剂性关节炎大鼠下丘脑HSP70的影响及保护机制研究[J]. 北京中医药大学学报,2005,28(4):86-89. doi: 10.3321/j.issn:1006-2157.2005.04.027 [17] Pipicz M,Kocsis G F,Sarvary-arantes L,et al. Low-dose endotoxin induces late preconditioning,increases peroxynitrite formation,and activates STAT3 in the rat heart[J]. Molecules (Basel,Switzerland),2017,22(3):E433. doi: 10.3390/molecules22030433 [18] Yuan G,Yu Y,Ji L,et al. Down-regulated receptor interacting protein 140 is involved in lipopolysaccharide-preconditioning-induced inactivation of kupffer cells and attenuation of hepatic ischemia reperfusion injury[J]. PloS One,2016,11(10):e0164217. doi: 10.1371/journal.pone.0164217 [19] Garcia-bonilla L,Brea D,Benakis C,et al. Endogenous protection from ischemic brain injury by preconditioned monocytes[J]. The Journal of Neuroscience:The Official Journal of the Society for Neuroscience,2018,38(30):6722-6736. doi: 10.1523/JNEUROSCI.0324-18.2018 [20] 侯婧瑛,于萌蕾,郭天柱,等. 缺氧预处理激活HIF-1α/MALAT1/VEGFA通路促进骨髓间充质干细胞生存和血管再生[J]. 中国组织工程研究,2021,25(7):985-990. doi: 10.3969/j.issn.2095-4344.2160 [21] 王婕,余丽梅. 炎症因子干预影响间充质干细胞的免疫调控特性[J]. 中国组织工程研究,2020,24(13):2108-2113. doi: 10.3969/j.issn.2095-4344.2054 [22] 王迪生,孔刘莎,王佳,等. 红细胞生成素预处理脂肪间充质干细胞移植治疗大鼠糖尿病肾病[J]. 中国组织工程研究,2019,23(9):1370-1376. doi: 10.3969/j.issn.2095-4344.1601 [23] 曲铁兵. 川芎嗪预处理促进骨髓间充质干细胞迁移的体外研究[D]. 杭州: 浙江中医药大学硕士学位论文, 2014. -





 下载:
下载: