Effects of Modulation of CDC42 Activity in Hippocampal Neurons on Memory and Anxiety Behaviors of Mice
-
摘要:
目的 建立动物模型研究海马神经元中细胞分裂周期蛋白42(Cell division cycle 42, CDC42)和认知缺陷之间的关系。 方法 包装携带CDC42突变体基因的腺病毒,通过显微注射操作使病毒感染健康小鼠海马脑区的兴奋性神经元,检测多种行为学范式下小鼠的认知表型。 结果 腺病毒能特异性的增强或降低CDC42蛋白在海马区兴奋性神经元的表达活性。CDC42蛋白活性的升高能促进小鼠24 h和96 h条件恐惧记忆的遗忘,同时能使小鼠表现出明显的焦虑行为。而抑制CDC42蛋白活性能促进条件恐惧记忆的维持。但是改变海马区CDC42蛋白活性不会影响小鼠的社交行为(P > 0.05)。 结论 海马区CDC42蛋白活性的改变能够影响小鼠的条件恐惧记忆及焦虑行为,揭示了CDC42蛋白与认知缺陷表型有着密切的联系。这一调控模型可能为精神分裂症等相关精神疾病研究模型的建立提供新的方法和思路,而CDC42有望成为治疗精神疾病记忆损伤或焦虑的一个新的分子靶点。 Abstract:Objective To establish an animal model to investigate the relationship between cell division cycle 42 (CDC42) and cognitive deficits in hippocampal neurons. Methods Adenovirus with CDC42 mutant gene was packaged to infect excitatory neurons in the hippocampus of healthy mice by microinjection. The cognitive phenotypes of mice under various behavioral patterns were detected. Results Adenovirus specifically enhanced or decreased the expression activity of CDC42 protein in excitatory neurons in the hippocampus. After the inhibition of CDC42 protein in the hippocampus, the conditioned memory of the mice decreased ,significantly at 24 hours and 96 hours, and enhancing CDC42 activity in the hippocampus made mice show an obvious anxiety behavior. However, neither enhancement nor inhibition of the CDC42 activity in the hippocampus had a significant effect on social behavior (P > 0.05). Conclusions The mice with altered CDC42 activity in hippocampal excitatory neurons may present a fear conditioning memory defect and significant anxiety behavior, this may suggest the relationship between cytoskeletal protein CDC42 and cognitive phenotypes. This regulatory model may provide new methods and ideas for the establishment of research models for mental disorders such as SZ and CDC42 is expected to become a new molecular target for the treatment of memory impairment or anxiety. -
Key words:
- CDC42 /
- Schizophrenia /
- Memory impairment /
- Anxiety
-
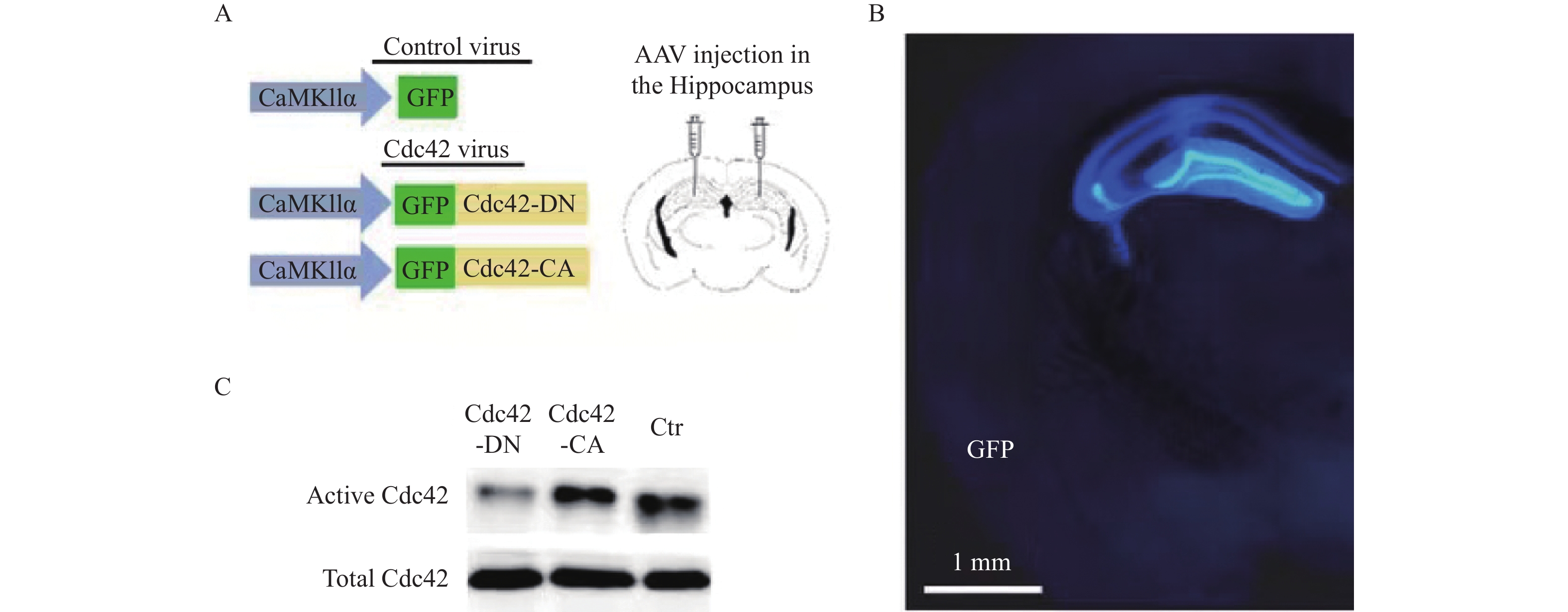
图 1 AAV-CaMKII-CDC42-GFP特异性地操纵海马区CDC42蛋白的活性
A:CDC42-AAV病毒载体构建示意图及立体定位注射示意图。CDC42-DN:CDC42负显性失活突变体;CDC42-CA:CDC42持续激活突变体;B:AAV病毒载体荧光信号在海马中表达;C:Western blot结果显示CDC42-DN能降低海马区CDC42蛋白的表达活性,而CDC42-CA能增强海马区CDC42蛋白的表达活性。
Figure 1. AAV-CaMKII-CDC42-GFP specifically regulated CDC42 activity in hippocampal neurons.
图 3 CDC42活性变化不影响小鼠的学习能力且新环境对恐惧记忆的表现无影响
A:小鼠进行恐惧记忆学习的训练模式示意图,训练结束后在原训练箱中马上测试(绿色)及马上放入新环境(context)中测试(红色);B:训练结束后,各组小鼠的学习能力无差异(绿色);C:新环境对各组实验小鼠的恐惧记忆无显著性地影响(红色)。绿色和红色框表示不同的环境。
Figure 3. CDC42 activity regulation did not affect the learning and the new environment did not affect the performance of fear memory in mice
图 5 调控海马神经元中CDC42蛋白的活性不影响小鼠社交记忆
A:小鼠社交行为范式示意图。训练阶段(A左图):实验小鼠(白色)放入箱中后和第一只陌生小鼠(黄色,被限制活动区域)进行10 min的自由探索;测试阶段:24 h后,往箱中放入熟悉小鼠(黄色)和第二只陌生小鼠(蓝色),更换熟悉小鼠的位置避免小鼠空间记忆对本实验结果的影响,放入实验小鼠检测其对另外两只小鼠的探索时间。B和C:CDC42-CA和CDC42-DN同正常小鼠相比,其社交记忆没有显著性差异。
Figure 5. Regulation of CDC42 activity in hippocampal neurons had no effect on social memory of mice
-
[1] Joober R,Rouleau G A,Lal S,et al. Neuropsychological impairments in neuroleptic-responder vs.-nonresponder schizophrenic patients and healthy volunteers[J]. Schizophr Res,2002,53(3):229-238. doi: 10.1016/S0920-9964(01)00279-1 [2] Bodnar M,Malla A,Joober R,et al. Cognitive markers of short-term clinical outcome in first-episode psychosis[J]. Br J Psychiatry,2008,193(4):297-304. doi: 10.1192/bjp.bp.107.040410 [3] Montreuil T,Bodnar M,Bertrand M C,et al. Social cognitive markers of short-term clinical outcome in first-episode psychosis[J]. Clin Schizophr Relat Psychoses,2010,4(2):105-114. doi: 10.3371/CSRP.4.2.2 [4] Brissos S,Dias V V,Balanza-Martinez V,et al. Symptomatic remission in schizophrenia patients:Relationship with social functioning,quality of life,and neurocognitive performance[J]. Schizophr Res,2011,129(2-3):133-136. doi: 10.1016/j.schres.2011.04.001 [5] Lepage M,Bodnar M,Bowie C R. Neurocognition:clinical and functional outcomes in schizophrenia[J]. Can J Psychiatry,2014,59(1):5-12. [6] Wright I C,Rabe-Hesketh S,Woodruff P W,et al. Meta-analysis of regional brain volumes in schizophrenia[J]. Am J Psychiatry,2000,157(1):16-25. doi: 10.1176/ajp.157.1.16 [7] Stepan-Buksakowska I,Szabo N,Horinek D,et al. Cortical and subcortical atrophy in Alzheimer disease:Parallel atrophy of thalamus and hippocampus[J]. Alzheimer Dis Assoc Disord,2014,28(1):65-72. doi: 10.1097/WAD.0b013e318299d3d6 [8] Gross R,Neria Y. Combat duty in Iraq and Afghanistan and mental health problems[J]. New England Journal of Medicine,2004,351(17):1798-1799. [9] Meyer-Lindenberg A,Poline J B,Kohn P D,et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia[J]. Am J Psychiatry,2001,158(11):1809-1817. doi: 10.1176/appi.ajp.158.11.1809 [10] Govek E E,Newey S E,Van A L. The role of the Rho GTPases in neuronal development[J]. Genes Dev,2005,19(1):1-49. [11] Luo L,Rho G T. Pases in neuronal morphogenesis[J]. Nat Rev Neurosci,2000,1(3):173-180. [12] Van Aelst L,Cline H T,Rho G T. Pases and activity-dependent dendrite development[J]. Curr Opin Neurobiol,2004,14(3):297-304. [13] Shuai Y,Lu B,Hu Y,et al. Forgetting is regulated through Rac activity in Drosophila[J]. Cell,2010,140(4):579-589. doi: 10.1016/j.cell.2009.12.044 [14] Zhang X,Li Q,Wang L,et al. Cdc42-dependent forgetting regulates repetition effect in prolonging memory retention[J]. Cell Rep,2016,16(3):817-825. doi: 10.1016/j.celrep.2016.06.041 [15] Dong T,He J,Wang S,et al. Inability to activate Rac1-dependent forgetting contributes to behavioral inflexibility in mutants of multiple autism-risk genes[J]. Proc Natl Acad Sci U S A,2016,113(27):7644-7649. doi: 10.1073/pnas.1602152113 [16] Hori K,Nagai T,Shan W,et al. Heterozygous disruption of autism susceptibility candidate 2 causes impaired emotional control and cognitive memory[J]. PLoS One,2015,10(12):e0145979. doi: 10.1371/journal.pone.0145979 [17] Hill J J,Hashimoto T,Lewis D A. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia[J]. Mol Psychiatry,2006,11(6):557-566. [18] Datta D,Arion D,Corradi J P,et al. Altered expression of CDC42 signaling pathway components in cortical layer 3 pyramidal cells in schizophrenia[J]. Biol Psychiatry,2015,78(11):775-785. doi: 10.1016/j.biopsych.2015.03.030 [19] Cetin A,Komai S,Eliava M,et al. Stereotaxic gene delivery in the rodent brain[J]. Nat Protoc,2006,1(6):3166-3173. doi: 10.1038/nprot.2006.450 [20] Prut L,Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors:A review[J]. Eur J Pharmacol,2003,463(1-3):3-33. [21] Udo H,Jin I,Kim J H,et al. Serotonin-induced regulation of the actin network for learning-related synaptic growth requires Cdc42,N-WASP,and PAK in Aplysia sensory neurons[J]. Neuron,2005,45(6):887-901. doi: 10.1016/j.neuron.2005.01.044 [22] Haditsch U,Leone D P,Farinelli M,et al. A central role for the small GTPase Rac1 in hippocampal plasticity and spatial learning and memory[J]. Mol Cell Neurosci,2009,41(4):409-419. doi: 10.1016/j.mcn.2009.04.005 [23] Kim I H,Wang H,Soderling S H,et al. Loss of Cdc42 leads to defects in synaptic plasticity and remote memory recall[J]. eLife,2014,3:e02839. [24] Hayashi-Takagi A,Takaki M,Graziane N,et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1[J]. Nat Neurosci,2010,13(3):327-332. doi: 10.1038/nn.2487 [25] Ramos-Miguel A,Barr A M,Honer W G. Spines,synapses,and schizophrenia[J]. Biol Psychiatry,2015,78(11):741-743. -






 下载:
下载: