Effect of Time Interval on the Outcome with Transarterial Chemoembolization Followed Microwave Ablation with Hepatocellular Carcinoma
-
摘要:
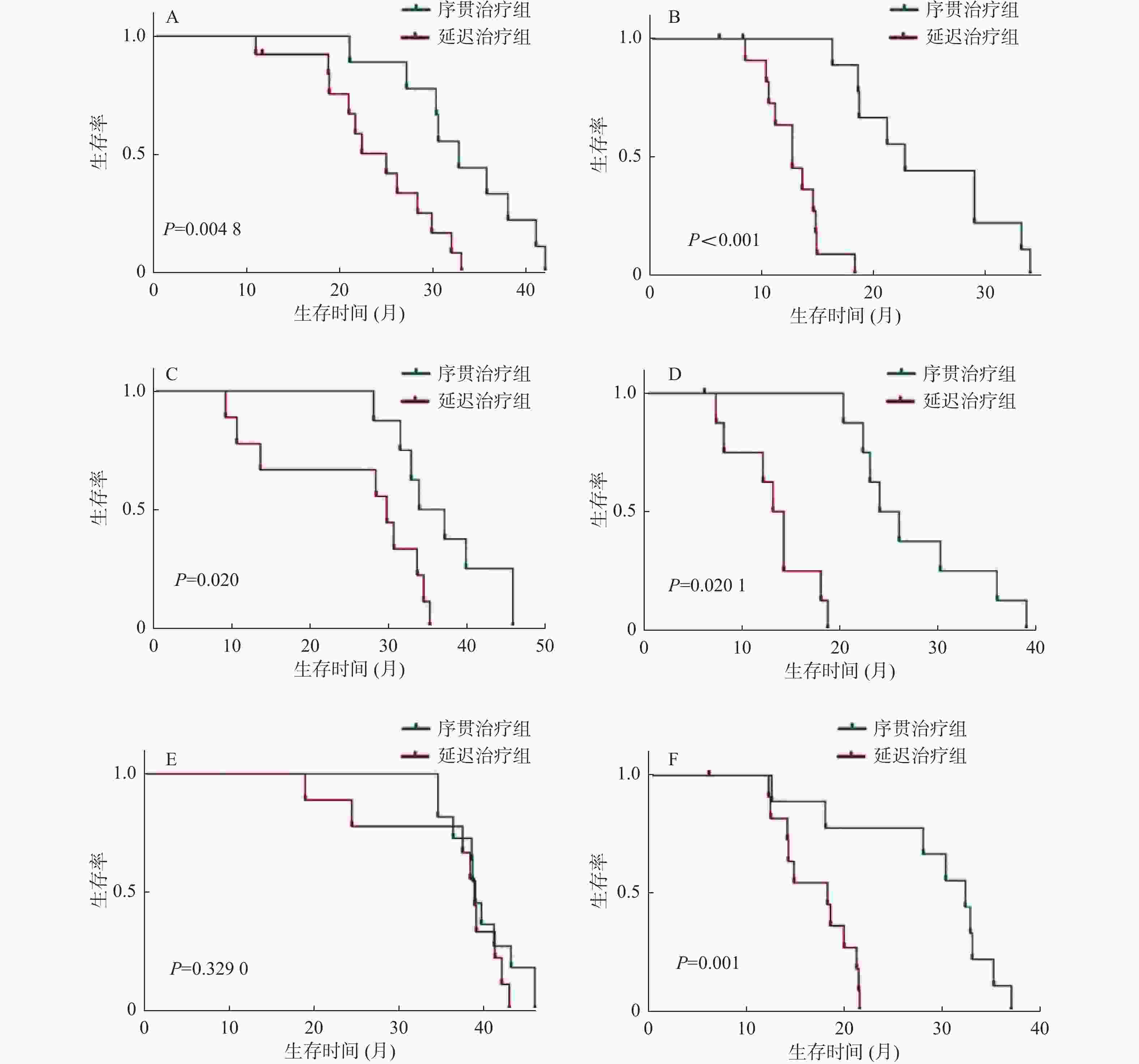
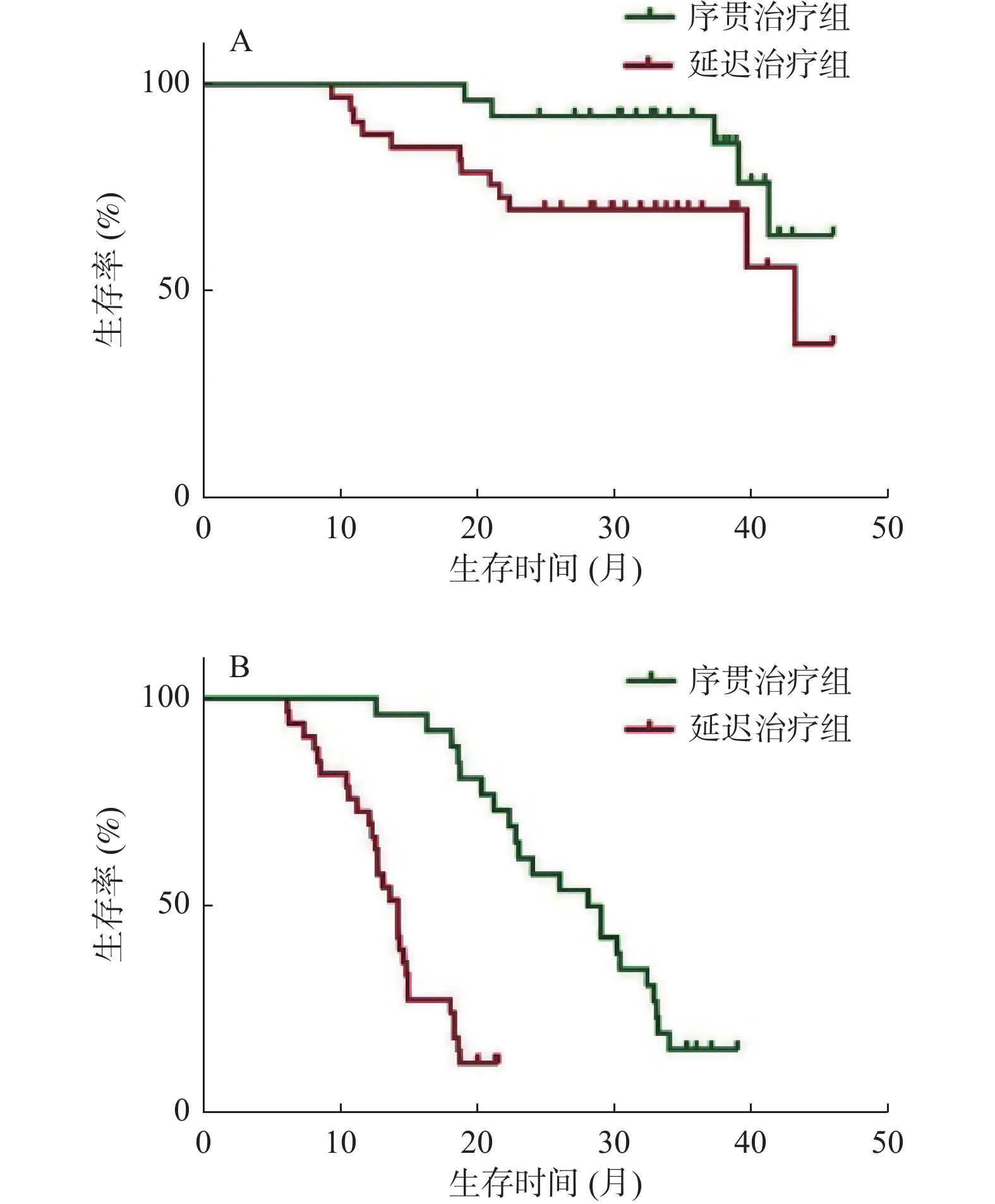
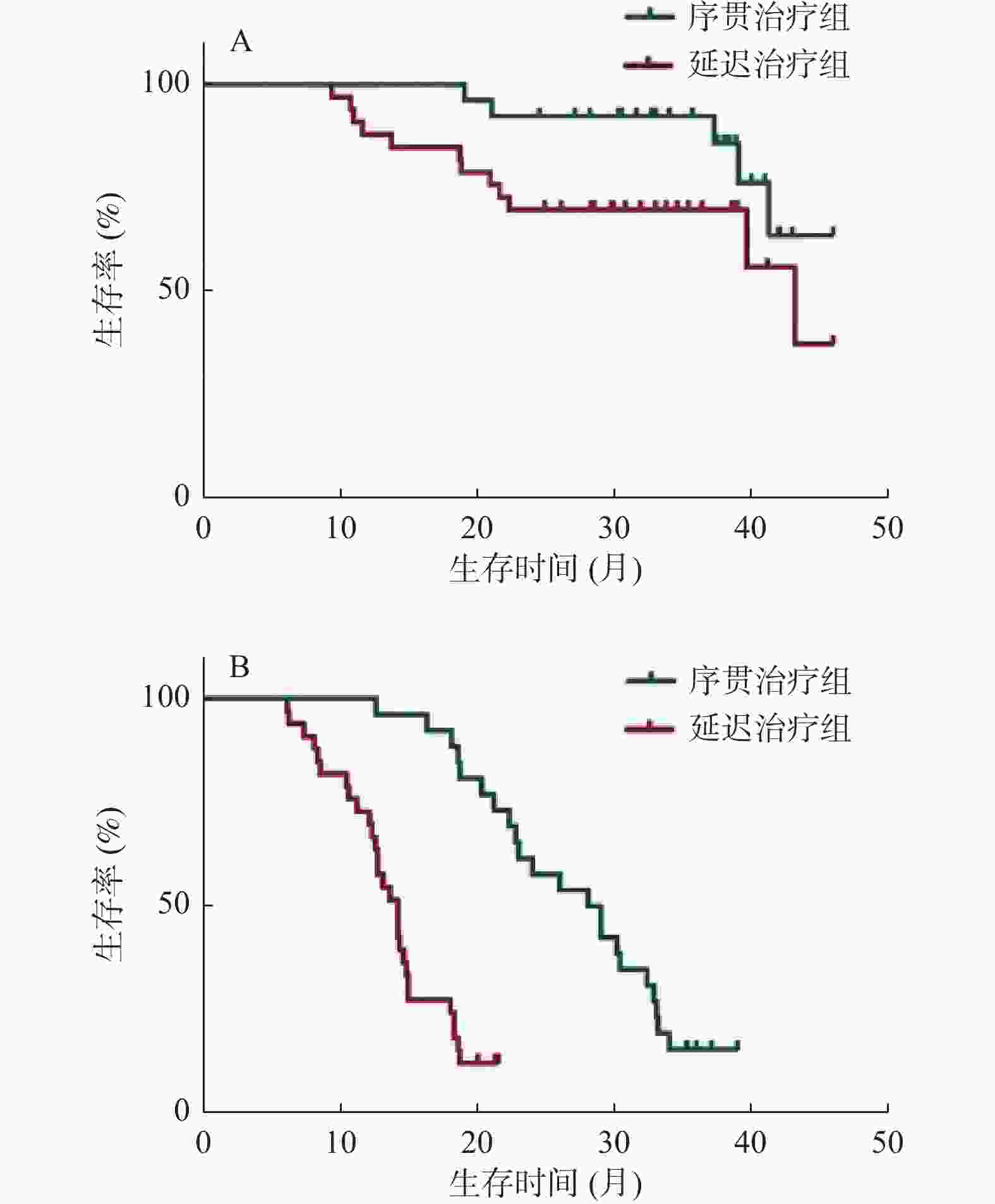
目的 分析时间间隔对经肝动脉化疗栓塞术(transarterial chemoembolization,TACE)后序贯微波消融(MWA)治疗肝细胞癌(hepatocellular carcinoma,HCC)的影响。 方法 回顾性分析2016年2月至2018年12月在昆明市第三人民医院接受TACE和MWA后复发的71例HCC患者,32例在TACE术后4周内序贯MWA治疗(序贯治疗组),39例在TACE术4周后接受延迟MWA治疗(延迟治疗组)。观察2组的生存期和无进展生存期,采用Kaplan-Meier法绘制生存曲线,使用多元Cox比例风险回归评估分析预后因素,对比2组的不良反应。 结果 序贯治疗组和延迟治疗组的中位生存期分别为35.3个月、29.1个月,序贯治疗组的无进展生存期为27个月,延迟治疗组的无进展生存期为13.8个月。序贯治疗组有更好的生存期(HR,0.601;95%CI 0.378~0.796;P = 0.037)和更长的无进展生存期(HR,0.779;95%CI 0.415~0.821;P = 0.004)。最大肿瘤直径、肿瘤个数和时间间隔是影响生存期和无进展生存期的因素,Child-Pugh分级是影响生存期的因素,但不会影响无进展生存期。 结论 在4周内实施TACE序贯MWA比超过4周实施延迟MWA治疗肝细胞癌,有更好的中位生存期和无进展生存期,且不增加不良反应。 Abstract:Objective To analyse the effect of time interval on the outcome of microwave ablation (MWA)treatment of hepatocellular carcinoma (HCC) after transarterial chemoembolization (TACE). Methods A retrospective analysis of recurrent 71 HCC patients after receiving TACE and MWA at the 3rd People’s Hospital of Kunming from February 2016 to December 2018; with 32 patients received sequential MWA treatment within 4 weeks after TACE (sequential Treatment group), and 39 patients received delayed MWA treatment (delayed Treatment group) 4 weeks after the completion of TACE, the overall survival (OS) and progression-free survival (PFS) of two groups were observed. Survival curve was plotted using Kaplan-Meier, the multiple Cox proportional hazard regression was used to analyze prognostic factors, compare adverse reactions between the two groups. Results The median OS of the sequential group and the delayed group were 35.3 months and 29.1 months, respectively. PFS in the sequential group was 27 months, and 13.8 months in the delayed group. The sequential group had better overall survival OS (HR, 0.601; 95%CI, 0.378-0.796; P = 0.037) and longer PFS (HR, 0.779; 95%CI, 0.415-0.821; P = 0.004). Maximum tumor diameter, number of tumors, and time interval are factors that affect OS and PFS. Child-Pugh classification is a factor that affects OS but does not affect PFS. Conclusion TACE plus sequential MWA within 4 weeks was more effective for treatment HCC than delayed MWA, and does not increase adverse reactions. -
表 1 肝癌患者的一般基线特征[(
$ \bar x \pm s $ )/n(%)]Table 1. Baseline characteristics of patients with HCC [(
$ \bar x \pm s $ )/n(%)]指标 序贯治疗组(n = 32) 延迟治疗组(n = 39) t/χ2 P 中位年龄(岁) 52.4 ± 2.63 54.8 ± 3.21 3.395 0.320 男/女 25(78.1)/7(21.9) 31(79.5)/8(20.5) 0.02 0.864 病原学 4.181 0.678 HBV感染 26(81.3) 33(84.6) HCV感染 6(18.7) 6(15.4) 抗病毒治疗 28(87.5) 35(89.7) 0.088 0.757 最大肿瘤直径(cm) 3.41 ± 1.3(1.5~10) 3.66 ± 1.1(1.5~8.7) 0.878 0.427 肿瘤数量 单发/多发 21(65.6)/11(34.4) 24(61.5)/15(38.5) 0.126 0.706 Child-Pugh分级A/B 24(72.7)/8(25) 30(76.9)/9(23.1) 0.036 0.814 AFP[μg/LT] 10.569 0.903 < 20 3(9.4) 2(5.1) 20~200 4(12.5) 6(15.4) ≥200 25(78.1) 31(79.5) PT(s) 12.8 ± 1.4 12.4 ± 1.5 1.152 0.299 HBV:乙型肝炎病毒;HCV:丙型肝炎病毒;AFP:甲胎蛋白;PT:凝血酶时间。 表 2 影响OS和PFS的单因素和多因素分析
Table 2. Univariate and multivariate analyses of factors that affected OS and PFS
指标 OS PFS 单变量 多变量 单变量 多变量 P HR(95% CI) P P HR(95% CI) P 年龄 0.527 1.013(0.742~1.311) 0.803 0.673 1.311(0.926~1.447) 0.625 性别 0.855 0.988(0.929~1.114) 0.426 0.961 0.979(0.851~1.736) 0.868 病原学 0.632 1.414(0.727~1.339) 0.551 0.704 1.214(0.837~1.123) 0.159 抗病毒治疗 0.771 0.665(0.913~1.127) 0.778 0.829 0.886(0.884~1.921) 0.421 最大肿瘤直径 0.001* 1.734(1.477~2.716) 0.041* 0.039* 2.127(1.327~2.846) 0.028* 肿瘤数量 0.001* 1.908(1.496~3.018) 0.006* 0.042* 2.016(1.522~3.018) 0.037* Child分级 0.041* 1.529(1.307~1.946) 0.047* 0.571 1.447(0.994~2.965) 0.068 PT 0.603 1.141(0.991~1.335) 0.629 0.806 1.213(0.992~3.471) 0.929 时间间隔 0.036* 1.307(1.138~2.086) 0.041* 0.029* 1.536(1.227~2.133) 0.026* OS:总生存期;PFS:无进展生存期;PT:凝血酶时间。*P < 0.05。 表 3 序贯组和延迟组术后并发症的情况[n(%)]
Table 3. Post-operative complications in patients who received TACE plus sequential MWA or TACE plus delayed MWA [n(%)]
组别 n 发热 腹痛 肝功异常 穿刺部位出血 χ2 P 序贯组 32 5(15.6) 9(28.1) 17(53.1) 1(3.1) 1.105 0.776 延迟组 39 5(12.8) 7(17.9) 17(43.6) 0(0.0) -
[1] Sung H,Ferlay J,Siegel R L,et al. Global Cancer Statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2021,71(3):209-249. doi: 10.3322/caac.21660 [2] Cho J Y,Han H S,Choi Y,et al. Association of remnant liver ischemia with early recurrence and poor survival after liver resection in patients with hepatocellular carcinoma[J]. JAMA Surg,2017,152(4):1-7. [3] Marrer J A,Kulik L M,Sirlin C B,et al. Diagnosis,staging,and management of hepatocellular carcinoma:2018 practice guidance by the American Association for the Study of Liver Diseases[J]. Hepatology,2018,68(2):723-750. doi: 10.1002/hep.29913 [4] Omata M,Cheng A L,Kokudo N,et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma:A 2017 update[J]. Hepatol Int,2017,11(4):317-370. doi: 10.1007/s12072-017-9799-9 [5] Chen Y P,Zhang J L,Zou Y,et al. Recent advances on polymeric beads or hydrogels as embolization agents forimproved transcatheter arterial ahemoembolization (TACE)[J]. Front Chem,2019,5(7):408. [6] Camma C,Schepis F,Orlando A,et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma:Meta-analysis of randomized controlled trials[J]. Radiology,2002,224(1):47-54. doi: 10.1148/radiol.2241011262 [7] Izzo F,Granata V,Grassi R,et al. Radiofrequency ablation and microwave ablation in liver tumors:An update[J]. Oncologist,2019,24(10):990-1005. doi: 10.1634/theoncologist.2018-0337 [8] Sheta E,Elkalla F,Elgharib M,et al. Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma:A randomized-controlled study[J]. Eur J Gastroenterol Hepatol,2016,28(10):1198-1203. doi: 10.1097/MEG.0000000000000688 [9] Schlachterman A,Craftww J R,Hilgendeldt E,et al. Current and future treatments for hepatocellular carcinoma[J]. World J Gastroenterol,2015,21(28):8478-8491. doi: 10.3748/wjg.v21.i28.8478 [10] Lencioni R,Llovet J M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma[J]. Semin in Liver Dis,2010,30(1):52-60. doi: 10.1055/s-0030-1247132 [11] 王贵强,王福生,成军,等. 慢性乙型肝炎防治指南(2015更新版)[J]. 中华肝脏病杂志,2015,23(12):888-905. doi: 10.3760/cma.j.issn.1007-3418.2015.12.002 [12] 魏来,侯金林. 丙型肝炎防治指南(2015年更新版)[J]. 中华实验和临床感染病杂志(电子版),2015,9(5):590-607. doi: 10.3877/cma.j.issn.1674-1358.2015.05.002 [13] Bodzin A S,Busuttil R W. Hepatocellular carcinoma:Advances in diagnosis,management,and long term outcome[J]. World J Hepatol,2015,7(9):1157-1167. doi: 10.4254/wjh.v7.i9.1157 [14] Makary M S,Khandpur U,Cloyd J M,et al. Locoregional therapy approaches for hepatocellular carcinoma:Recent advances and management strategies[J]. Cancers (Basel),2020,12(7):1914. doi: 10.3390/cancers12071914 [15] Zhu F,Rhim H. Thermal ablation for hepatocellular carcinoma:What's new in 2019[J]. Chin Clin Oncol,2019,8(6):58. doi: 10.21037/cco.2019.11.03 [16] Dong B W,Zhang J,Liang P,et al. Sequential pathological and immunologic analysisof percutaneous microwave coagulation therapy of hepatocellular carcinoma[J]. Int J Hyperthermia,2003,19(2):119-133. doi: 10.1080/0265673021000017154 [17] Guo H,Chen B,Li W,et al. Percutaneous microwave coagulation therapy:A promising therapeutic method for breaking the barrier of the intertumor heterogeneity[J]. J Healthc Eng,2021,11(19):7773163. [18] Zhang J,Dong B,Liang P,et al. Significance of changes in local immunity in patients with hepatocellular carcinoma after percutaneous microwave coagulation therapy[J]. Chin Med J (Engl),2002,115(9):1367-1371. [19] Zhang E L, Yang F, Wu Z B, et al, Therapeutic efficacy of percutaneous microwave coagulation versus liver resection for single hepatocellular carcinoma ≤3 cm with Child-Pugh A cirrhosis[J]. Eur J Surg Oncol, 2016, 42(5): 690-697. [20] Li J,Tao H S,Li J,et al. Effect of severity of liver cirrhosis on surgical outcomes of hepatocellular carcinoma after liver resection and microwave coagulation[J]. Front Oncol,2021,6(11):745615. [21] Ryu T,Takmi Y,Wada Y,et al. Hepatic resection versus operative microwave ablation for single hepatocellular carcinoma ≤5 cm:A propensity score-matched analysis[J]. Surgery,2019,166(3):254-262. doi: 10.1016/j.surg.2019.05.007 [22] Lee S,Kang T W,Cha D I,et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma:Propensity score analyses of long-term outcomes[J]. J Hepatol,2018,69(1):70-78. doi: 10.1016/j.jhep.2018.02.026 [23] Leung U,Kuk D,Dangelica M I,et al. Long-term outcomes following microwave ablation for liver malignancies[J]. Br J Surg,2015,102(1):85-91. [24] Zhang N N,Lu W,Cheng X J,et al. High-powered microwave ablation of larger hepatocellular carcinoma:Evaluation of recurrence rate and factors related to recurrence[J]. Clin Radiol,2015,70(11):1237-1243. doi: 10.1016/j.crad.2015.06.092 [25] Zhang T T,Luo H C,Cui X,et al. Ultrasound-guided percutaneous microwave ablation treatment of initial recurrent hepatocellular carcinoma after hepatic resection:Long-term outcomes[J]. Ultrasound Med Biol,2015,41(9):2391-2399. doi: 10.1016/j.ultrasmedbio.2015.04.019 [26] Dong Z R,Zhang P F,Wang C H,et al. Postoperative adjuvant transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond the Milan criteria:A retrospective analysis[J]. Am J Cancer Res,2015,5(1):450-457. [27] Ke Q,Xiang F,Xiao C,et al. Exploring the clinical value of preoperative serum gamma-glutamyl transferase levels in the management of patients with hepatocellular carcinoma receiving postoperative adjuvant transarterial chemoembolization[J]. BMC Cancer,2021,21(1):1117. doi: 10.1186/s12885-021-08843-z -





 下载:
下载: