Optimal Total Nedaplatin Dose of Concurrent Chemotherapy in Patients with Stage III-IVA Nasopharyngeal Carcinoma
-
摘要:
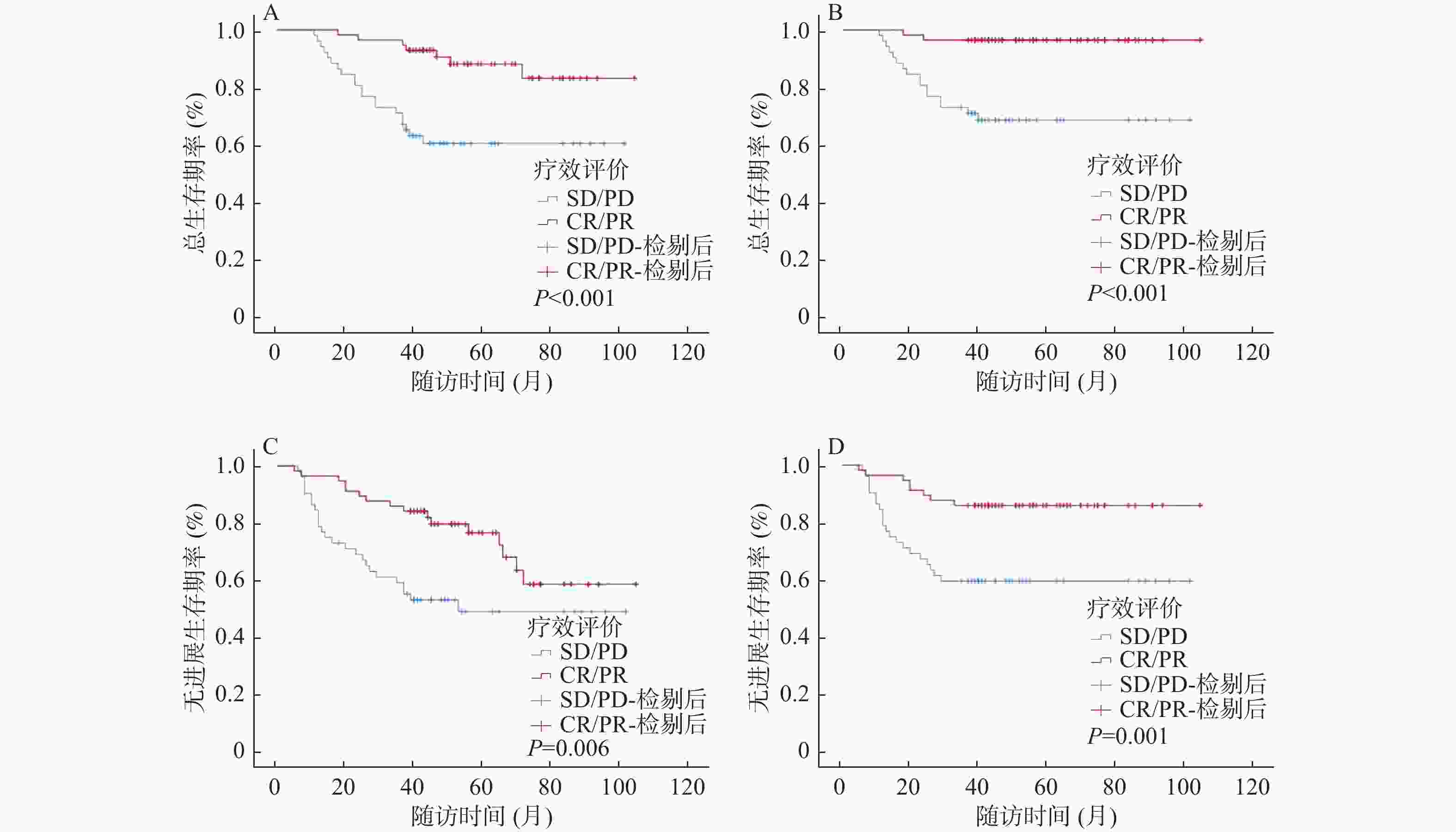
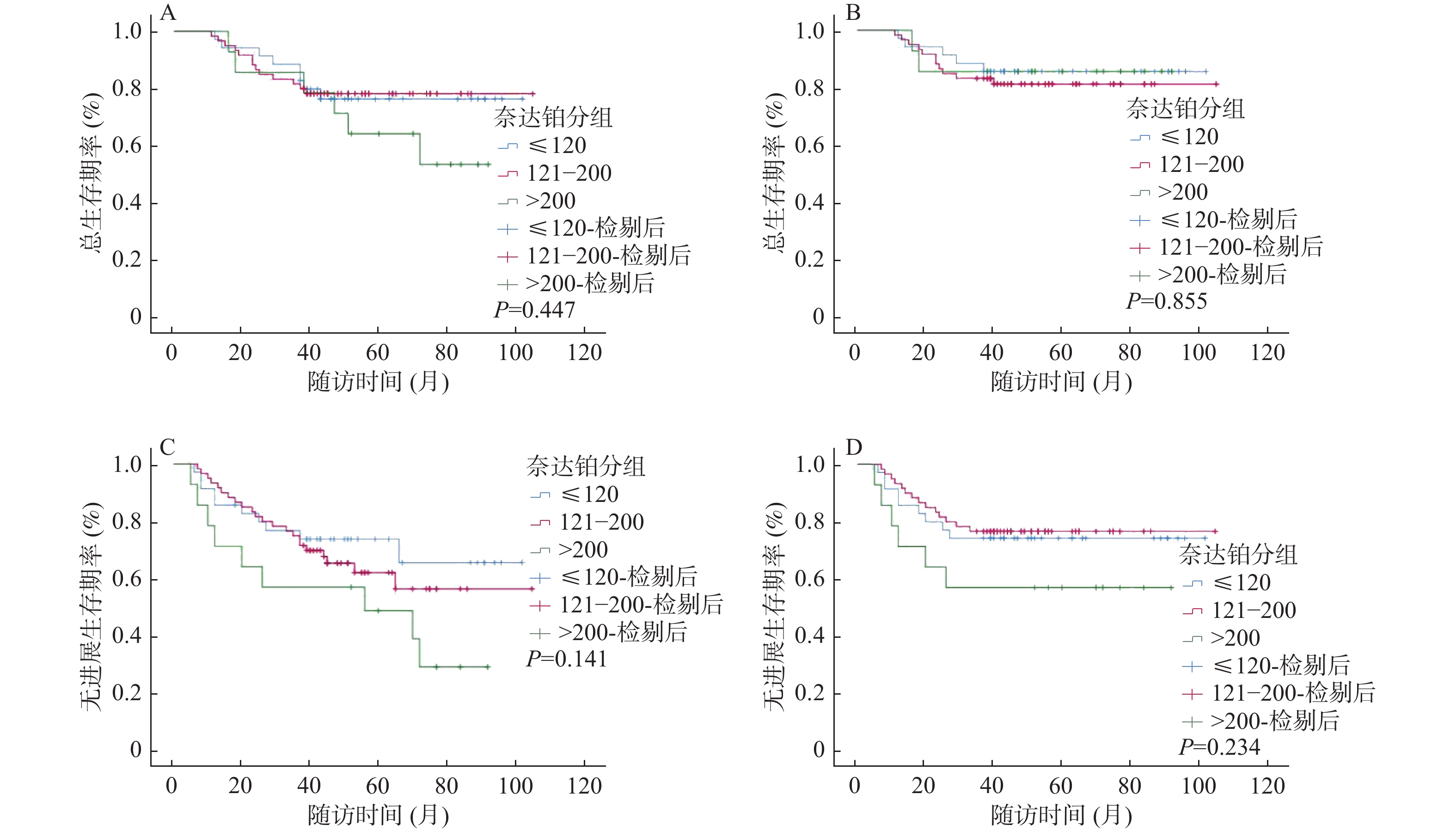
目的 探索诱导化疗后的III-IVA期的鼻咽癌患者放疗同期化疗时最适累积奈达铂总量,分析同期奈达铂总量对诱导化疗后的鼻咽癌患者的预后影响。 方法 收集2013年1月至2018年12月首诊于云南省肿瘤医院共109例接受IC+奈达铂CCRT,根据CND四分位数将奈达铂组患者分为低(≤120 mg/m2)、中(121-200 mg/m2)、高剂量( > 200 mg/m2)3个组别,再根据肿瘤诱导化疗后反应将奈达铂组患者分别分为CR/PR和SD/PD 2个亚组,分析统计诱导化疗后不同累积奈达铂总量对患者的预后的影响,以期得到最适CND。 结果 CND低、中、高剂量组各项生存率,差异均无统计学意义(P > 0.05);根据CR/PR、SD/PD分组进行生存分析,累积OS率分别为77.2%、50.0%,3 a OS率98.2%、62.9%,累积PFS率63.2%、45.0%,3 a PFS率77.2%、54.8%,CR/PR组的各生存率优于SD/PD组(P < 0.05);根据CND低、中、高剂量分别对2个亚组进行分析,CR/PR亚组的累积OS率为93.3%、93.8%、60.0%(P = 0.046),3 a OS率100.0%、93.8%、100.0%(P > 0.05),累积PFS率86.7%、78.1%、30.0%(P = 0.007),3 a PFS率93.3%、90.6%、60.0%(P = 0.017),低剂量组及中剂量组在OS率、PFS率、3 a PFS率方面较高剂量组显示出显著优势;SD/PD亚组中各生存率,差异无统计学意义(P > 0.05);在多因素分析中,IC后的疗效评价(CR/PR&SD/PD)是III-IVA期鼻咽癌患者OS、3 a OS、PFS、3 a PFS的独立预后因素(P < 0.05)。 结论 III-IVA期鼻咽癌患者IC后CR/PR患者的生存获益远优于SD/PD的患者,IC后的肿瘤反应(CR/PR&SD/PD)是III-IVA期鼻咽癌患者的独立预后因素。III-IVA期鼻咽癌患者在IC后CR/PR的亚组中,接受低、中CND的患者生存率较高剂量组改善显著,对于该类患者来说CND似乎不需要超过200 mg/m2 即可获得较好生存结果。 Abstract:Objective To explore the most appropriate total cumulative nedaplatin dose during chemotherapy for patients at the III-IVA stage after induced chemotherapy, and to analyze the effect of the total amount of nedaplatin on the prognosis of nasopharyngeal carcinoma patients after induction chemotherapy. Methods In this study, a total of 109 patients who were first diagnosed at Yunnan Cancer Hospital from 2013 to 2018 received induction chemotherapy (IC) plus concurrent chemoradiotherapy (CCRT). According to the CND quartile, the patients were divided into three groups: low (≤120 mg/m2), medium (121-200 mg/m2) and high dose ( > 200 mg/m2). According to the response after tumor induction chemotherapy, the Nedaplatin group was divided into CR/PR and SD/PD subgroups, respectively. The effects of different cumulative dose of nedaplatin on the prognosis of patients after induction chemotherapy were analyzed to obtain the optimal CND. Results The difference in survival rates between the low, medium and high dose groups of CND were not statistically significant (P > 0.05). According to the survival analysis of CR/PR and SD/PD groups, the cumulative OS rates were 77.2% and 50.0%, the 3-year OS rates were 98.2% and 62.9%, the cumulative PFS rates were 63.2% and 45.0%, and the 3 a PFS rates were 77.2% and 54.8%, respectively. The survival rate of CR/PR group was better than that of SD/PD group (P < 0.05). According to the low, medium and high dose of CND, the two subgroups were analyzed respectively. The cumulative OS rate of CR/PR subgroup was 93.3%, 93.8% and 60.0% (P = 0.046), and the 3 year OS rates were 100.0%, 93.8% and 100.0% (P > 0.05). The cumulative PFS rates were 86.7%, 78.1% and 30.0% (P = 0.007), and the 3 year PFS rates were 93.3%, 90.6% and 60.0% (P = 0.017). The low-dose and medium-dose groups showed significant advantages in OS rate, PFS rate and 3year PFS rate. There was no significant difference in survival rate among SD/PD subgroups (P > 0.05). In multivariate analysis, efficacy evaluation after IC (CR/PR&SD/PD) was an independent prognostic factor for OS, 3 year OS, PFS, and 3 year PFS in patients with stage III-IVA nasopharyngeal carcinoma (P < 0.05). Conclusion The survival benefit of CR/PR patients with stage III-IVA nasopharyngeal carcinoma after IC is much better than that of SD/PD patients. Tumor response after IC (CR/PR&SD/PD) is an independent prognostic factor for stage III-IVA nasopharyngeal carcinoma patients. In the post-IC CR/PR subgroup of patients with stage III-IVA nasopharyngeal carcinoma, the survival rate of patients receiving low and medium CND improved significantly in the higher dose group. It seems that CND does not need to exceed 200 mg/m2 to achieve better survival results for these patients. -
表 1 奈达铂组不同CND患者基线特征[n(%)]
Table 1. Baseline characteristics of different CND patients in the nedaplatin group [n(%)]
特征 ≤120 mg/m2 121~200 mg/m2 > 200 mg/m2 统计量 P 性别 Fisher = 2.987 0.224 男 26(34.2) 38(50.0) 12(15.8) 女 9(27.3) 22(66.7) 2(6.0) T分期 Fisher = 2.723 0.863 T1 4(30.8) 9(69.2) 0(0.0) T2 9(30.0) 17(56.7) 4(13.3) T3 14(35.0) 20(50.0) 6(15.0) T4 8(30.8) 14(53.8) 4(15.4) N分期 Fisher = 2.301 0.924 N0 1(33.3) 1(33.3) 1(33.3) N1 7(36.8) 10(52.6) 2(10.6) N2 21(30.4) 39(56.) 9(13.0) N3 6(33.3) 10(55.6) 2(11.1) 总分期 χ2 = 0.490 0.785 III期 22(31.4) 40(57.2) 8(11.4) IVA期 13(33.3) 20(51.3) 6(15.4) 病理类型 Fisher = 0.392 0.851 低分化鳞癌 30(31.6%) 53(55.8%) 12(12.6) 未分化癌 5(35.7%) 7(50.0%) 2(14.3) 诱导化疗方案 Fisher = 4.165 0.328 TPF 2(20.0) 5(50.0) 3(30.0) TP 32(34.8) 50(54.3) 10(10.9) GP 1(14.3) 5(71.4%) 1(14.3) 表 2 奈达铂组患者Cox分析(%)
Table 2. Cox analysis of patients in nedaplatin group (%)
因素 危险比HR(95%CI) P OS 年龄(岁≤47岁& > 47岁) 0.570(0.252~1.288) 0.177 性别(男&女) 0.640(0.231~1.777) 0.392 T分期(T1-2&T3-4) 1.138(0.409~3.167) 0.804 N分期(N0-1&N2-3) 2.872(0.870~9.480) 0.083 总分期(III期&IVA期) 0.224(0.089~0.563) 0.001* 疗效评价(CR/PR&SD/PD) 0.145(0.056~0.372) < 0.001* 病理类型(低分化鳞癌&未分化癌) 2.792(0.669~11.655) 0.159 累积奈达铂总量(mg/m2)(≤120+121-200& > 200) 2.236(0.716~6.985) 0.166 累积奈达铂总量(mg/m2)(≤120& > 200+121-200) 1.192(0.477~2.980) 0.707 3 a OS 龄(岁≤47岁& > 47岁) 1.211(0.467~3.145) 0.694 性别(男&女) 0.866(0.275~2.723) 0.806 T分期(T1-2&T3-4) 1.261(0.399~3.980) 0.693 N分期(N0-1&N2-3) 7.990(0.954~66.925) 0.055 总分期(III期&IVA期) 0.591(0.202~1.724) 0.335 疗效评价(CR/PR&SD/PD) 0.068(0.014~0.321) 0.001* 病理类型(低分化鳞癌&未分化癌) 3.791(0.565~25.459) 0.170 累积奈达铂总量(mg/m2)(≤120+121-200& > 200) 0.771(0.131~4.533) 0.773 累积奈达铂总量(mg/m2)(≤120& > 200+121-200) 1.606(0.550~4.691) 0.386 PFS 年龄(岁≤47岁& > 47岁) 0.834(0.441~1.577) 0.576 性别(男&女) 0.638(0.290~1.406) 0.265 T分期(T1-2&T3-4) 0.900(0.408~1.984) 0.793 N分期(N0-1&N2-3) 1.250(0.515~3.037) 0.793 总分期(III期&IVA期) 0.439(0.218~0.882) 0.021* 疗效评价(CR/PR&SD/PD) 0.324(0.167~0.626) 0.001* 病理类型(低分化鳞癌&未分化癌) 2.056(0.725~5.835) 0.176 累积奈达铂总量(mg/m2)(≤120+121-200& > 200) 1.787(0.758~4.213) 0.185 累积奈达铂总量(mg/m2)(≤120& > 200+121-200) 1.573(0.731~3.382) 0.246 3 a PFS 年龄(岁≤47岁& > 47岁) 1.028(0.487~2.171) 0.942 性别(男&女) 0.859(0.353~2.094) 0.738 T分期(T1-2&T3-4) 0.710(0.267~1.890) 0.493 N分期(N0-1&N2-3) 0.817(0.309~2.157) 0.683 总分期(III期&IVA期) 0.407(0.177~0.931) 0.033* 疗效评价(CR/PR&SD/PD) 0.220(0.092~0.524) 0.001* 病理类型(低分化鳞癌&未分化癌) 2.205(0.679~7.161) 0.188 累积奈达铂总量(mg/m2)(≤120+121-200& > 200) 2.515(0.877~7.210) 0.086 累积奈达铂总量(mg/m2)(≤120& > 200+121-200) 1.042(0.447~2.428) 0.924 *P < 0.05。 -
[1] Wang C Q,He J. Ubiquitous distribution of epstein-barr virus and the highly uneven distribution of nasopharyngeal carcinoma[J]. Chin Med J (Engl),2016,129(20):2506-2507. doi: 10.4103/0366-6999.191827 [2] Kai Y,Arimura H,Toya R,et al. Comparison of rigid and deformable image registration for nasopharyngeal carcinoma radiotherapy planning with diagnostic position PET/CT[J]. Jpn J Radiol,2020,38(3):256-264. [3] Chen C Y,Lu T X,Zhao C,et al. Effect analysis of radiotherapy alone for T3 ~ T4N0 ~ N3 nasopharyngeal carcinoma[J]. Chinese Journal of Radiation Oncology,2006,15(2):77-80. [4] Sun Y,Li W F,Chen N Y,et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma:A phase 3,multicentre,randomised controlled trial[J]. Lancet Oncol,2016,17(11):1509-1520. doi: 10.1016/S1470-2045(16)30410-7 [5] Cao S M,Yang Q,Guo L,et al. Neoadjuvant chemotherapy followed by concurrent chemo-radiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma:A phase III multicentre randomised controlled trial[J]. Eur J Canc (Oxford,England,1990),2017,75:14-23. [6] Guo S S,Tang L Q,Zhang L,et al. The impact of the cumulative dose of cisplatin during concurrent chemoradiotherapy on the clinical outcomes of patients with advanced-stage nasopharyngeal carcinoma in an era of intensity- modulated radiotherapy[J]. BMC Cancer,2015,15:977. [7] Ang K K. Concurrent radiation chemotherapy for locally advancedhead and neck carcinoma:Are we addressing burning subjects[J]. J ClinOncol,2004,22(23):4657-4659. [8] Peng H,Chen L,Zhang,Y,et al. Prognostic value of the cumulativecisplatin dose during concurrent chemoradiotherapy in locoregionallyadvanced nasopharyngeal carcinoma:A secondary analysis of aprospective phase III clinical trial[J]. Oncologist,2016,21(11):1369-1376. doi: 10.1634/theoncologist.2016-0105 [9] Loong H H,Ma B B Y,Leung S F,et al. Prognostic significance of thetotal dose of cisplatin administered during concurrent chemoradio-therapy in patients with locoregionallyadvanced nasopharyngeal car-cinoma[J]. Radiother Oncol,2012,104(3):300-304. [10] Tang L Q,Chen D P,Chen L,et al. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II-IVB nasopharyngeal carcinoma:An open-label,non-inferiority,randomised phase 3 trial[J]. Lancet Oncol,2018,19(4):461-473. doi: 10.1016/S1470-2045(18)30104-9 [11] 吴鹏,赵玉梅,陈冬梅,等. 奈达铂与顺铂联合同期调强放疗治疗局部晚期鼻咽癌随机对照试验的Meta分析[J]. 肿瘤学杂志,2019,25(2):107-115. [12] Deng C,Zhang N,Jiang S,et al. Nedaplatin-based chemotherapy or cisplatin-based chemotherapy combined with intensity-modulated radiotherapy achieve similar efficacy for stage II-IVa nasopharyngeal carcinoma patients[J]. Sci Rep,2022,12(1):11978. doi: 10.1038/s41598-022-16216-0 [13] Sun X M,Su S F,Chen C Y,et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma:An analysis of survival and treatment toxicities[J]. Radiotherapy and Oncology,2014,110(3):398-403. doi: 10.1016/j.radonc.2013.10.020 [14] Liu L T,Tang L Q,Chen Q Y,et al. The prognostic value of plasma Epstein-Barrviral DNA and tumor response to neoadjuvant chemotherapy in advanced-stage nasopharyngeal carcinoma[J]. Int J Radiat Oncol Biol Phys,2015,93(4):862-869. doi: 10.1016/j.ijrobp.2015.08.003 [15] Peng H,Chen L,Zhang Y,et al. The tumour response to inductionchemotherapy has prognostic value for long-term survival outcomes afterintensity-modulated radiation therapy in nasopharyngeal carcinoma[J]. Sci Rep,2016,6:24835. doi: 10.1038/srep24835 [16] Liu S L,Sun X S,Yan J J,et al. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients based on induction chemotherapy response[J]. Radiother Oncol,2019,137:83-94. [17] Peng H,Chen L,Li W F,et al. Tumor response to neoadjuvantchemotherapy predicts long-term survival outcomes in patients withlocoregionally advanced nasopharyngeal carcinoma:A secondaryanalysis of a randomized phase 3 clinical trial[J]. Cancer,2017,123(9):1643-1652. doi: 10.1002/cncr.30520 [18] Lv J W,Qi Z Y,Zhou G Q,et al. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients receiving additional induction chemotherapy[J]. Cancer Sci,2018,109(3):751-763. doi: 10.1111/cas.13474 [19] Wei W,Huang Z,Li S,et al. Pretreatment Epstein-Barr virus DNA load and cumulative cisplatin dose intensity affect long-term outcome of nasopharyngeal carcinoma treated with concurrent chemotherapy:Experience of an institute in an endemic area[J]. Oncol Res Treat,2014,37(3):88-95. -





 下载:
下载: