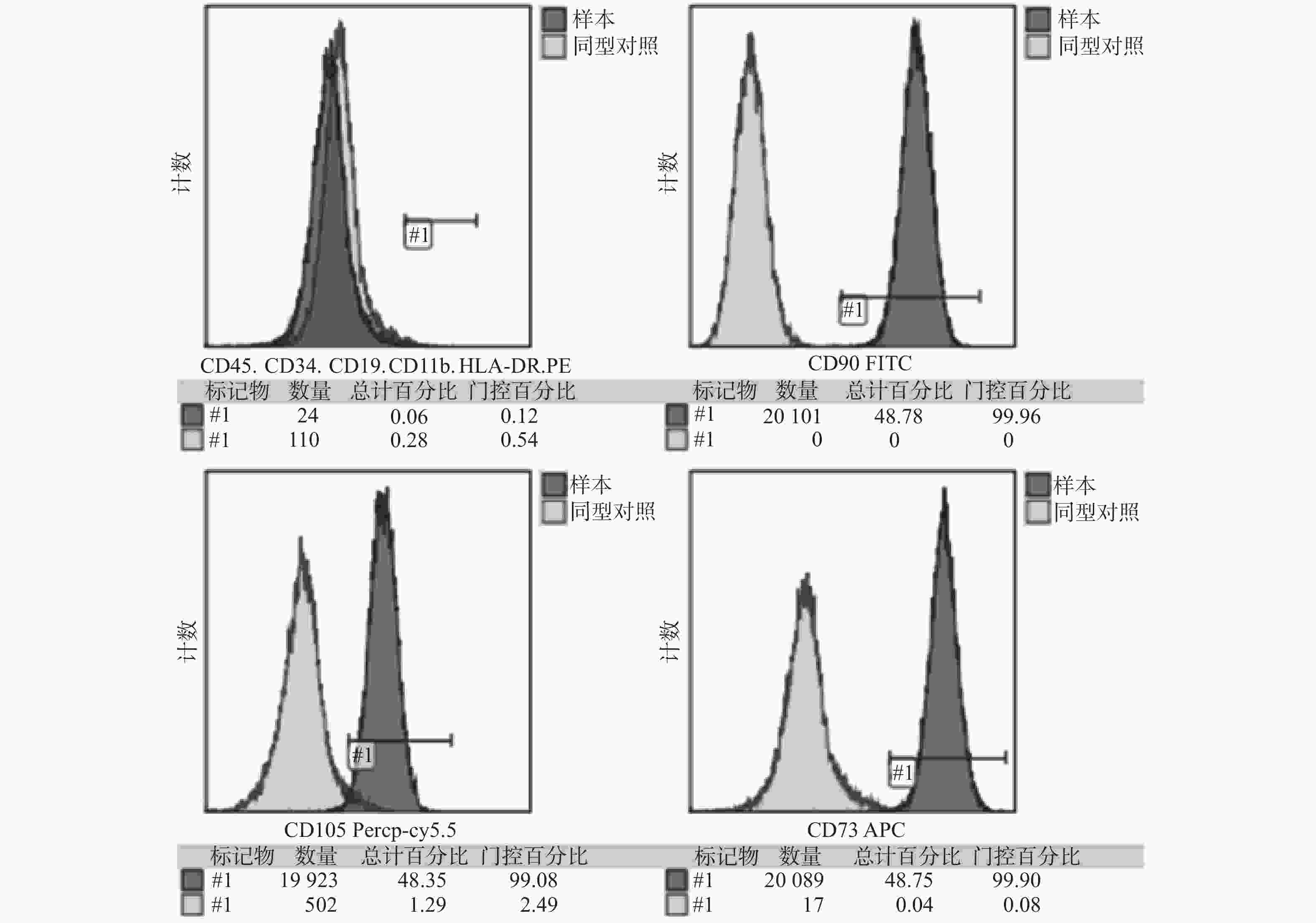
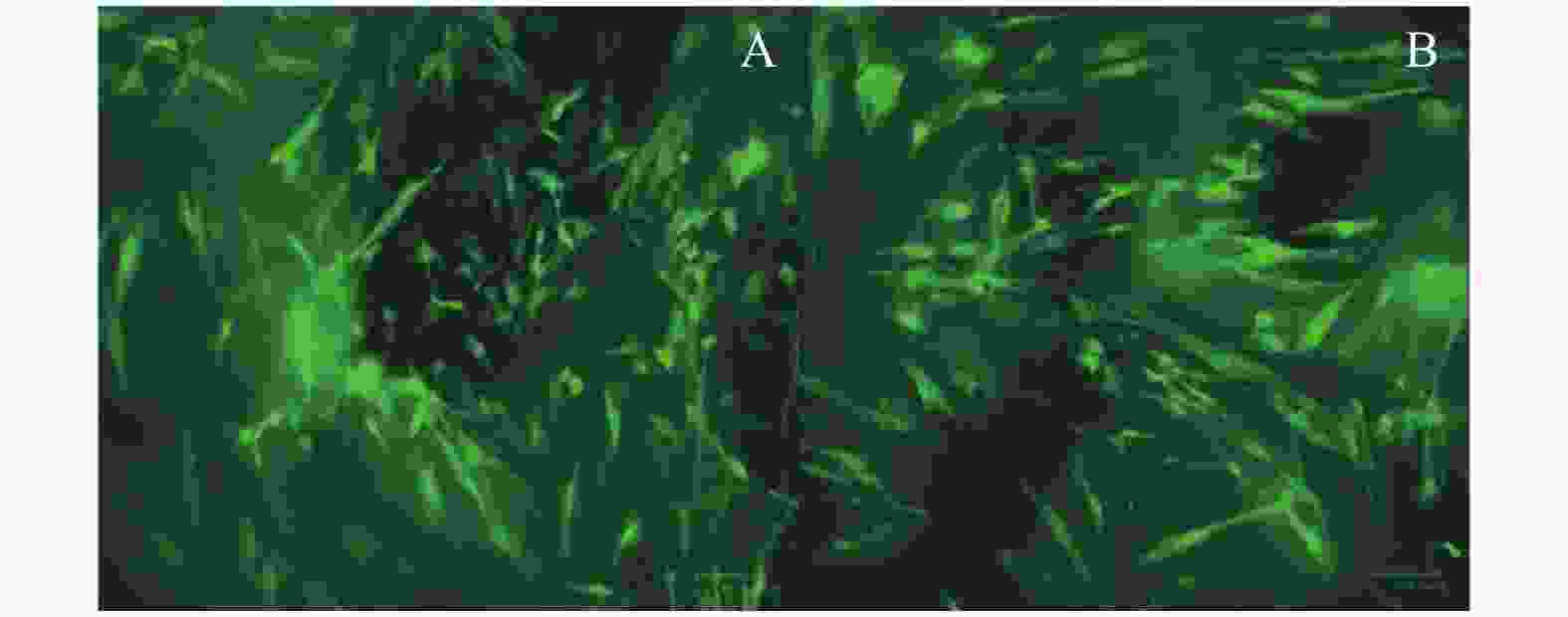
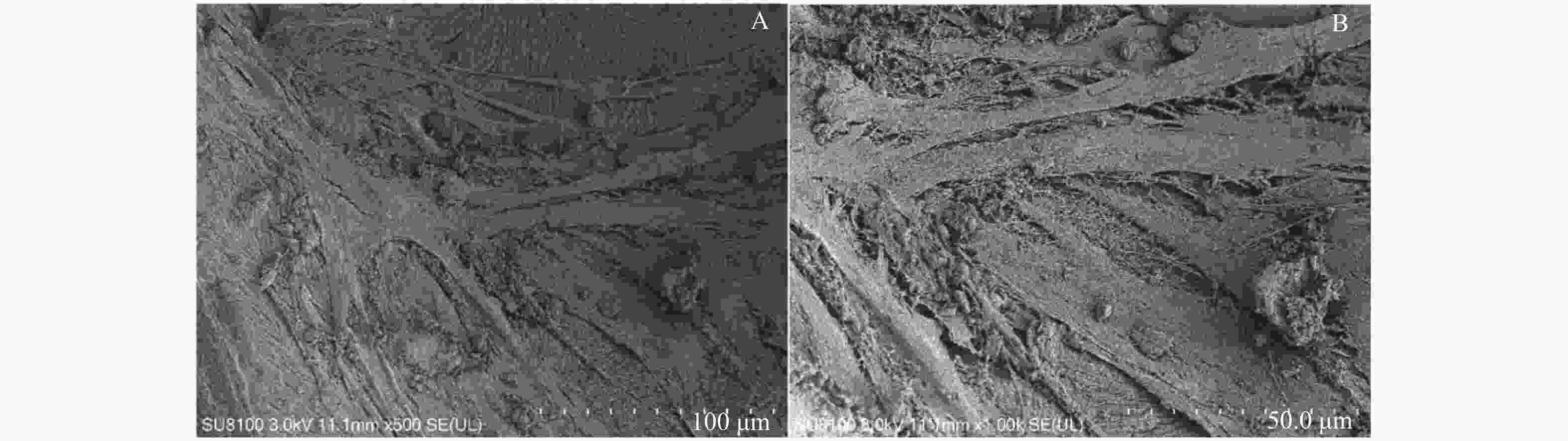
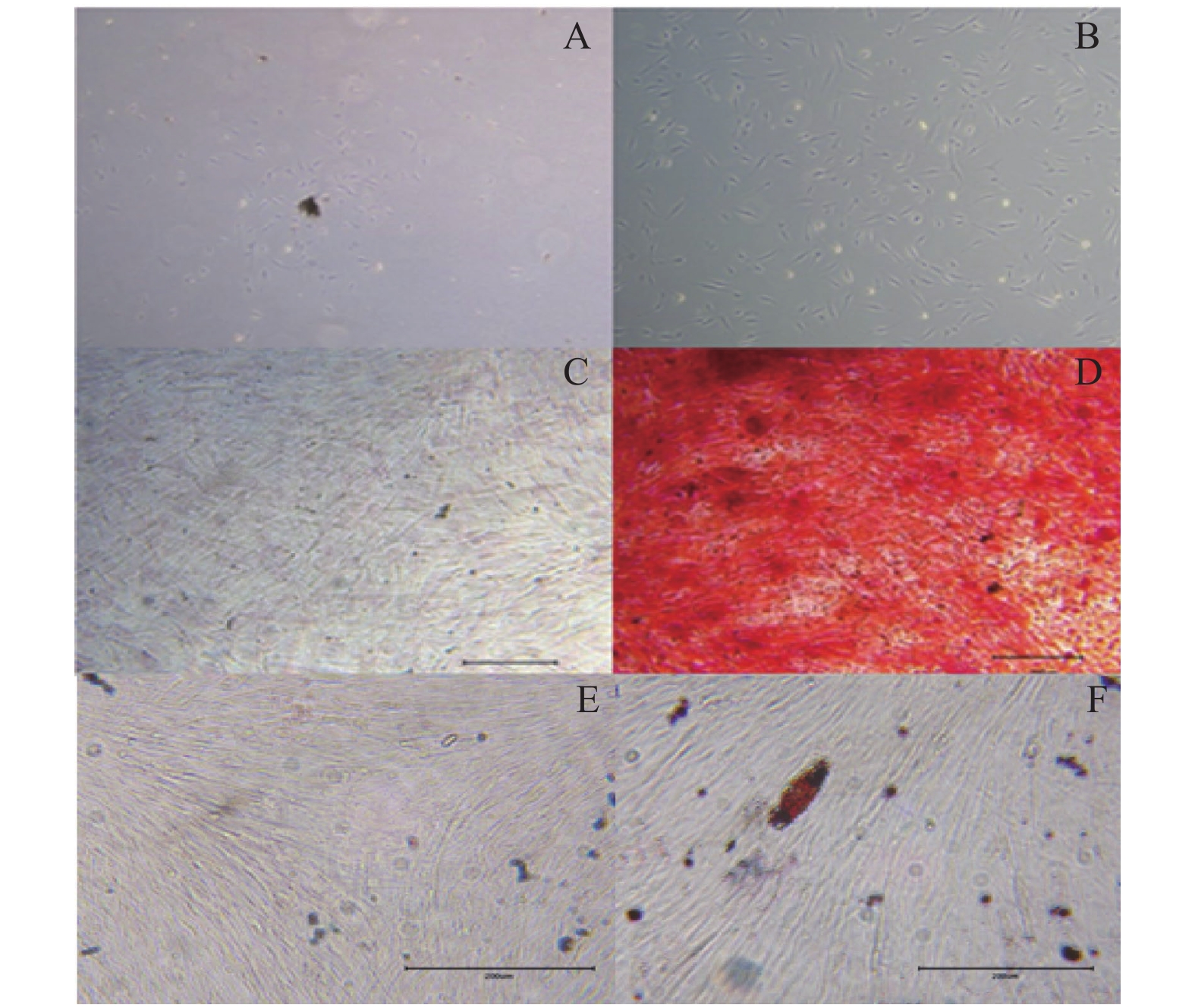
|
[1]
|
Le Thieu M K,Homayouni A,Haeren L R,et al. Impact of simultaneous placement of implant and block bone graft substitute: an in vivo peri-implant defect model[J]. Biomater Res,2021,25(1):43-52. doi: 10.1186/s40824-021-00245-3
|
|
[2]
|
Shiu S T,Lee W F,Chen S M,et al. Effect of different bone grafting materials and mesenchymal stem cells on bone regeneration: A micro-computed tomography and histomorphometric study in a rabbit calvarial defect model[J]. Int J Mol Sci,2021,22(15):8101-8115. doi: 10.3390/ijms22158101
|
|
[3]
|
Shi X,Mao J,Liu Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications[J]. Stem Cells Transl Med,2020,9(4):445-464. doi: 10.1002/sctm.19-0398
|
|
[4]
|
Tsutsui T W. Dental pulp stem cells: advances to applications[J]. Stem Cells Cloning,2020,13:33-42.
|
|
[5]
|
Gendviliene I,Simoliunas E,Alksne M,et al. Effect of extracellular matrix and dental pulp stem cells on bone regeneration with 3D printed PLA/HA composite scaffolds[J]. Eur Cell Mater,2021,41:204-215. doi: 10.22203/eCM.v041a15
|
|
[6]
|
Tanikawa D Y S,Pinheiro C C G,Almeida M C A,et al. Deciduous Dental Pulp Stem Cells for Maxillary Alveolar Reconstruction in Cleft Lip and Palate Patients[J]. Stem Cells Int,2020,2020:6234167.
|
|
[7]
|
Gil L F,Nayak V V,Benalcazar Jalkh E B,et al. Laddec(R) versus Bio-Oss(R): The effect on the healing of critical-sized defect - Calvaria rabbit model[J]. J Biomed Mater Res B Appl Biomater,2022,110(12):2744-2750. doi: 10.1002/jbm.b.35125
|
|
[8]
|
Li Y,Zhou W,Li P,et al. Comparison of the osteogenic effectiveness of an autogenous demineralised dentin matrix and Bio-Oss(R) in bone augmentation: a systematic review and meta-analysis[J]. Br J Oral Maxillofac Surg,2022,60(7):868-876. doi: 10.1016/j.bjoms.2022.03.009
|
|
[9]
|
Dai Y,Xu J,Han X H,et al. Clinical efficacy of mineralized collagen (MC) versus anorganic bovine bone (Bio-Oss) for immediate implant placement in esthetic area: a single-center retrospective study[J]. BMC Oral Health,2021,21(1):390-398. doi: 10.1186/s12903-021-01752-4
|
|
[10]
|
Shamsoddin E,Houshmand B,Golabgiran M. Biomaterial selection for bone augmentation in implant dentistry: A systematic review[J]. J Adv Pharm Technol Res,2019,10(2):46-50. doi: 10.4103/japtr.JAPTR_327_18
|
|
[11]
|
RAPONE B,INCHINGOLO A D,TRASARTI S,et al. Long-Term Outcomes of Implants Placed in Maxillary Sinus Floor Augmentation with Porous Fluorohydroxyapatite (Algipore((R)) FRIOS((R))) in Comparison with Anorganic Bovine Bone (Bio-Oss((R))) and Platelet Rich Plasma (PRP): A Retrospective Study[J]. J Clin Med,2022,11(9):2491-2503. doi: 10.3390/jcm11092491
|
|
[12]
|
Iaquinta M R,Martini F,D'agostino A,et al. Stem cell fate and immunomodulation promote bone regeneration via composite bio-oss((R))/avitene(TM) biomaterial[J]. Front Bioeng Biotechnol,2022,10:873814. doi: 10.3389/fbioe.2022.873814
|
|
[13]
|
Kosinski M,Figiel-dabrowska A,Lech W,et al. Bone defect repair using a bone substitute supported by mesenchymal stem cells derived from the umbilical cord[J]. Stem Cells Int,2020,2020:1321283.
|
|
[14]
|
Jiang Y H,Shang Y,Zou D H,et al. [Effect of rat allogeneic BMSCs-Bio-Oss-bFGF compound on tooth extraction healing: a micro-CT study][J]. Shanghai Kou Qiang Yi Xue,2022,31(1):38-43.
|
|
[15]
|
Zhou Q,Yu B H,Liu W C,et al. BM-MSCs and bio-oss complexes enhanced new bone formation during maxillary sinus floor augmentation by promoting differentiation of BM-MSCs[J]. In Vitro Cell Dev Biol Anim,2016,52(7):757-771. doi: 10.1007/s11626-015-9995-7
|
|
[16]
|
Xu X,Fang K,Wang L,et al. Local application of semaphorin 3a combined with adipose-derived stem cell sheet and anorganic bovine bone granules enhances bone regeneration in type 2 diabetes mellitus rats[J]. Stem Cells Int,2019,2019:2506463.
|
|
[17]
|
Chen Y,Huang H,Li G,et al. Dental-derived mesenchymal stem cell sheets: a prospective tissue engineering for regenerative medicine[J]. Stem Cell Res Ther,2022,13(1):38-52. doi: 10.1186/s13287-022-02716-3
|
|
[18]
|
Alksne M,Kalvaityte M,Simoliunas E,et al. Dental pulp stem cell-derived extracellular matrix: autologous tool boosting bone regeneration[J]. Cytotherapy,2022,24(6):597-607. doi: 10.1016/j.jcyt.2022.02.002
|
|
[19]
|
Davies O G,Cooper P R,Shelton R M,et al. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue,bone marrow and dental pulp[J]. J Bone Miner Metab,2015,33(4):371-382. doi: 10.1007/s00774-014-0601-y
|
|
[20]
|
Lee Y C,Chan Y H,Hsieh S C,et al. Comparing the osteogenic potentials and bone regeneration capacities of bone marrow and dental pulp mesenchymal stem cells in a rabbit calvarial bone defect model[J]. Int J Mol Sci,2019,20(20):5015-5024. doi: 10.3390/ijms20205015
|
|
[21]
|
Lorusso F,Inchingolo F,Dipalma G,et al. Synthetic scaffold/dental pulp stem cell (DPSC) tissue engineering constructs for bone defect treatment: an animal studies literature review[J]. Int J Mol Sci,2020,21(24):9765-9784. doi: 10.3390/ijms21249765
|
|
[22]
|
Wang F,Li Q,Wang Z. A comparative study of the effect of Bio-Oss((R)) in combination with concentrated growth factors or bone marrow-derived mesenchymal stem cells in canine sinus grafting[J]. J Oral Pathol Med,2017,46(7):528-536. doi: 10.1111/jop.12507
|
|
[23]
|
Qin R,Cui Z,Zhou H,et al. Effect of lentivirus-mediated BMP2 from autologous tooth on the proliferative and osteogenic capacity of human periodontal ligament cells[J]. J Periodontal Res,2022,57(4):869-879. doi: 10.1111/jre.13025
|
|
[24]
|
Zhou C,Zhang D,Zou J,et al. Substrate Compliance Directs the Osteogenic Lineages of Stem Cells from the Human Apical Papilla via the Processes of Mechanosensing and Mechanotransduction[J]. ACS Appl Mater Interfaces,2019,11(29):26448-26459. doi: 10.1021/acsami.9b07147
|
|
[25]
|
Shahabipour F,Oskuee R K,Shokrgozar M A,et al. CRISPR/Cas9 mediated GFP-human dentin matrix protein 1 (DMP1) promoter knock-in at the ROSA26 locus in mesenchymal stem cell for monitoring osteoblast differentiation[J]. J Gene Med,2020,22(12):e3288.
|





 下载:
下载: