Correlation of Metastatic and Prognostic Factors in 318 Cases of Primary Gastric Cancer
-
摘要:
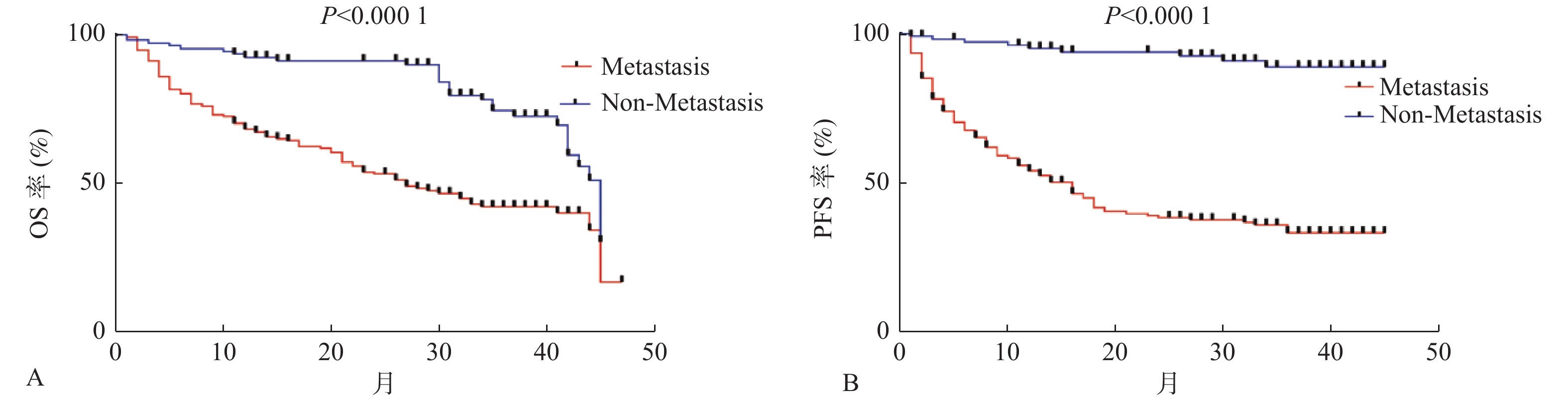
目的 探索并分析原发性胃癌(gastric cancer,GC)患者转移及预后的影响因素,寻找可早期预测转移的临床指标。 方法 回顾性研究云南省肿瘤医院2018年1月至2021年12月期间收治的经病理确诊为原发性GC的318例患者的临床病理资料,根据是否发生转移分为转移组(213例)和未转移组(105例)。分析各临床病理因素与GC转移及预后的关系,并探索各影响因素对转移的诊断效能。 结果 (1)生存分析提示转移组中位总生存期(overall survival,OS)及平均无进展生存期(progression-free survival,PFS)分别为27个月和22.16个月,未转移组中位OS及平均PFS分别为45个月和41.98个月,2组间OS及PFS,差异均有统计学意义(P < 0.0001);(2)多因素分析提示浸润深度 > 黏膜层、未接受化疗及糖类抗原-125(carbohydrate antigen-125,CA-125)≥14.4 KU/L是GC患者发生转移的独立危险因素( P < 0.05);(3)绘制受试者工作特征曲线(receiver operating characteristic curve,ROC曲线)提示与单指标或双指标联合相比,浸润深度、前白蛋白和CA-125三指标联合后对预测GC患者是否发生转移具有更精确的敏感性及特异性;(4)Log-rank检验及COX多因素分析提示化疗总周期<8周期及CA-125≥14.4 KU/L是转移患者OS的独立危险因素,原发灶未接受手术治疗、初诊合并转移、病程中合并远处转移及CA-125≥14.4 KU/L是转移患者PFS的独立危险因素,差异均有统计学意义( P < 0.05)。 结论 GC患者一旦发生转移则预后极差,临床中应重视转移的早期发现及诊治,多指标联合对于诊断GC转移具有较精确的敏感性及特异性,提示对GC患者应进行积极评估,制定个体化治疗方案,以期尽早诊治转移灶,为改善患者预后,延长生存期,提高生活质量提供一定的理论基础。 Abstract:Objective To explore the influencing factors of metastasis and prognosis in patients with primary gastric cancer (GC), and trying to find out the clinical indicators for early prediction of metastasis. Methods A retrospective study was conducted on the clinicopathological data of 318 patients with primary GC who were admitted to Yunnan Cancer Hospital from January 2018 to December 2021 and were divided into 2 groups: metastasis group (213 cases) and non-metastasis group (105 cases) according to whether metastasis occurred. The relationship between clinicopathological factors and GC metastasis and prognosis was analyzed, and the diagnostic efficacy of these factors for GC metastasis was explored. Results (1) The median overall survival (OS) and average progression-free survival (PFS) were 27 months and 22.16 months respectively in the metastasis group. While the median OS and average PFS in the non-metastasis group were 45 months and 41.98 months respectively. The differences in OS and PFS between these two groups were statistically significant (P < 0.0001). (2) Multivariate analysis suggested that infiltration depth > mucosal layer, no chemotherapy and carbohydrate antigen-125 (CA-125)≥14.4KU/L were independent risk factors for GC patients with metastasis ( P < 0.05). (3) The drawing of receiver operating characteristic curve (ROC curve) suggested that the combination of infiltration depth, prealbumin and CA-125 had more accurate sensitivity and specificity for predicting GC patients’ metastasis than the combination of double single or indexs. (4) Log-rank test and COX multivariate analysis suggested that total chemotherapy cycle < 8 cycles and CA-125≥14.4KU/L were independent risk factors for OS in patients with metastasis. The independent risk factors for PFS in patients with metastasis were no surgical treatment in the primary lesion, combined with metastasis at initial diagnosis, combined with distant metastasis during the course of disease and CA-125≥14.4KU/L, with statistically significant differences ( P < 0.05). Conclusions The prognosis of GC patients with metastasis is truly poor, clinical attention should be paid more attention to the early detection, diagnosis and treatment. The combination of multiple indexs has been proven to be more accurate in sensitivity and specificity for the diagnosis of GC metastasis, suggesting that GC patients should be actively evaluated and individualized treatment should be developed to diagnose and treat after metastasis as soon as possible, to provide a theoretical basis for improving patients’ prognosis, prolonging survival and improving life treatment. -
Key words:
- Gastric cancer /
- Clinical characteristics /
- Metastasis /
- Prognosis
-
表 1 临床一般资料与GC转移的单因素分析[n(%)]
Table 1. Univariate analysis of clinical general data and GC metastasis [n(%)]
变量 合计 转移组 非转移组
χ2 P 总人数 318(100.0) 213(66.98) 105(33.02) 性别 0.693 0.453 男 208(65.41) 136(63.85) 72(68.57) 女 110(34.59) 77(36.15) 33(31.43) 年龄(岁) 0.719 0.342 <45 54(16.98) 33(15.49) 21(20.00) ≥45 264(83.02) 180(84.51) 84(80.00) KPS评分 0.167 0.718 <90 39(12.26) 25(11.74) 14(13.33) ≥90 279(87.74) 188(88.26) 91(86.67) BMI(kg/m2) 0.679 <24 239(75.16) 162(76.06) 77(73.33) 0.279 ≥24 79(24.84) 51(24.68) 28(26.67) NRS评分 <0.0001 1.00 <3 243(76.42) 163(77.06) 80(76.19) ≥3 75(23.58) 49(23.04) 24(22.86) 原发灶 5.139 0.743 胃窦 151(47.48) 97(45.54) 54(51.43) 其他 167(52.52) 116(54.46) 51(48.57) 病理分型 0.291 0.655 腺癌 255(80.19) 169(79.34) 86(81.90) 其他 63(19.81) 44(20.66) 19(18.10) 浸润深度 44.141 <0.0001* ≤黏膜层 67(21.07) 23(10.80) 44(41.90) >黏膜层 234(73.58) 181(84.98) 53(50.48) 分化程度 1.611 0.204 高-中分化 38(11.95) 22(10.34) 16(15.24) 其他 280(88.68) 191(89.67) 89(84.76) 是否合并糖代谢紊乱 7.706 0.006* 是 46(14.47) 39(18.31) 7(6.67) 否 212(66.67) 174(81.69) 98(93.33) 原发灶是否接受手术 15.177 <0.0001* 是 228(71.70) 138(64.79) 90(85.71) 否 90(28.30) 75(35.21) 15(14.29) 是否化疗 24.354 <0.0001* 是 251(78.93) 185(86.85) 66(62.86) 否 67(21.07) 28(13.15) 39(37.14) 总化疗周期 0.35 0.581 <8 周 207(65.09) 164(77.00) 59(56.19) ≥8 周 44(13.84) 37(17.37) 10(9.52) HER2 3.616 0.706 阴性 135(42.45) 151(70.89) 56(53.33) 阳性 123(38.68) 34(15.96) 10(9.52) Epstein-Barr病毒感染 2.52 0.118 是 58(49.69) 42(19.72) 16(15.24) 否 171(53.77) 104(48.83) 67(63.81) 幽门螺旋杆菌感染 0.739 0.419 是 73(22.96) 50(23.47) 23(21.90) 否 95(29.87) 59(27.70) 34(32.38) *P < 0.05。 表 2 实验室指标与GC转移的单因素分析[M(P25,P75)/n(%)]
Table 2. Univariate analysis of laboratory indexes and GC metastasis [M(P25,P75)/n(%)]
变量 转移组(n = 213) 非转移组(n = 105) χ2 P 白细胞(109/L) 6.09(4.84,7.72) 5.93(4.96,7.56) 0.681 0.496 LYMP(109/L) 1.65(1.26,2.08) 1.82(1.38,2.21) 2.099 0.036* 单核细胞(109/L) 0.34(0.26,0.49) 0.33(0.26,0.43) 0.766 0.444 中性粒细胞(109/L) 3.77(2.94,5.53) 3.49(2.65,4.98) 1.588 0.112 血小板(109/L) 266(206,318.5) 241(194,327.5) 1.281 0.20 中性粒细胞/白细胞比值 0.63(0.56,0.74) 0.60(0.53,0.68) 2.602 0.009* NLR 2.58(1.72,5.98) 2.15(1.48,4.16) 1.979 0.048* LWR 0.28(0.18,0.35) 0.31(0.24,0.38) 2.919 0.004* PLR 157.75(110.24,218.2) 134(97.32,191.09) 2.23 0.026* 营养指标 HB(g/L) 129(111,146) 140(121.5,157.5) 3.654 <0.001* TP(g/L) 69(64,74) 70(67,75) 2.348 0.019* PA(g/L) 217.7(169.4,278.25) 254.3(217.95,307.95) 4.423 <0.0001* ALB(g/L) 43(39,46) 45(42,48) 3.931 <0.0001* AGR 1.63(1.45,1.87) 1.74(1.55,1.93) 2.549 0.011* GLU(mmol/L) 5.06(4.59,5.72) 4.83(4.37,5.33) 2.729 0.006* 总胆固醇(mmol/L) 4.35(3.67,5.01) 4.35(4.98,5.04) 1.317 0.188 低密度脂蛋白(mmol/L) 2.62(2.12,3.28) 2.68(2.27,3.1) 0.365 0.715 HDL(mmol/L) 1.08(0.93,1.27) 1.17(0.99,1.45) 2.925 0.003* 甘油三酯(mmol/L) 1.3(0.99,1.82) 1.35(1.02,1.7) 0.238 0.812 凝血功能指数 PT(s) 12.7(12.3,13.3) 12.5(12.1,12.9) 2.877 0.004* TT(s) 15.6(14.8,16.3) 16(15.4,17.03) 3.542 <0.001* 活化部分凝血活酶时间(s) 36.3(33.95,36.1) 36.25(33.98,38.7) 0.393 0.694 FIB(g/L) 3.87(3.2,4.85) 3.39(2.83,3.95) 3.67 <0.001* 肿瘤标志物 CEA(μg/L) 2.9(1.62,7.59) 2.29(1.51,4.07) 2.469 0.014* CA-199(KU/L) 11.76(5.97,27.28) 9.01(5.08,19.24) 1.993 0.046* CA-724(KU/L) 3.22(1.47,13.16) 3.16(1.35,9.59) 1.224 0.221 CA-125(KU/L) 15.6(9.99,34.17) 10.97(7.8,14.12) 5.228 <0.0001* CA-242(KU/L) 4.2(1.26,12.84) 4.07(1,10.67) 0.379 0.704 *P < 0.05。 表 3 GC患者转移的多因素分析
Table 3. Multivariate analysis of metastasis in GC patients
变量 B SE Wald P Exp(B) 95%CI 是否合并糖代谢紊乱 0.026* 0.591 0.002* 0.965 1.026 (0.322,3.271) 浸润深度 −1.257 0.393 10.207 0.001* 0.285 (0.132,0.615) 原发灶是否接受手术 −0.475 0.425 1.249 0.264 0.622 (0.271,1.43) 是否化疗 0.926 0.415 4.990 0.025* 2.525 (1.12,5.69) LYMP −0.090 0.316 0.081 0.776 0.914 (0.492,1.698) NWR 0.617 0.344 3.214 0.073 0.999 (0.944,3.638) NLR −0.055 0.039 2.002 0.157 0.947 (0.878,1.021) LWR −2.057 2.116 0.945 0.331 1.853 (0.002,8.094) PLR <0.001* 0.002* <0.001* 0.994 0.128 (0.996,1.004) HB −0.001* 0.007* 0.020* 0.886 1.00 (0.985,1.013) TP −0.032* 0.035* 0.869 0.351 0.968 (0.905,1.036) PA 0.004* 0.003* 1.540 0.215 1.004 (0.998,1.006) ALB 0.011 0.056 0.039* 0.844 1.011 (0.906,1.128) AGR 0.015* 0.107 0.020* 0.887 1.015 (0.823,1.253) GLU −0.260 0.158 2.704 0.10 0.771 (0.566,1.051) HDL 0.734 0.481 2.331 0.127 2.082 (0.812,5.341) PT −0.251 0.231 1.175 0.278 0.778 (0.494,1.225) TT 0.227 0.136 2.773 0.096 1.254 (0.961,1.638) FIB −0.077 0.101 0.580 0.446 0.926 (0.759,1.129) CEA <0.001* 0.002* 0.057 0.811 1.00 (0.996,1.003) CA-199 <0.001* 0.000* 0.238 0.626 1.00 (0.999,1.001) CA-125 −0.023* 0.010* 4.992 0.025* 0.978 (0.958,0.997) *P < 0.05。 表 4 单个临床指标对GC转移的诊断效能
Table 4. The diagnostic effectiveness of a single clinical index for GC metastasis
变量 AUC(95%CI) P 最佳截断值 灵敏度 特异度 约登指数 是否合并糖代谢紊乱 0.558(0.493~0.621) 0.091 1.5 0.184 0.933 0.116 浸润深度 0.67(0.601~0.74) <0.0001* 1.5 0.887 0.454 0.341 原发灶是否接受手术 0.605(0.541~0.668) 0.002* 3.5 0.352 0.857 0.209 是否化疗 0.602(0.552~0.668) 0.001* 1.5 0.869 0.371 0.24 LYMP 0.572(0.506~0.638) 0.036* 1.82 0.638 0.505 0.143 NWR 0.59(0.524~0.656) 0.009 0.61 0.596 0.552 0.148 NLR 0.568(0.50~0.636) 0.048* 1.765 0.736 0.41 0.147 LWR 0.601(0.535~0.666) 0.004* 0.35 0.768 0.394 0.18 PLR 0.577(0.51~0.644) 0.026* 142.26 0.582 0.59 0.172 HB 0.626(0.559~0.692) <0.001* 145.5 0.746 0.476 0.222 TP 0.581(0.516~0.646) 0.019* 65.5 0.338 0.838 0.176 PA 0.652(0.59~0.715) <0.0001* 216.9 0.498 0.781 0.279 ALB 0.635(0.571~0.699) <0.0001* 45.5 0.728 0.486 0.214 AGR 0.588(0.522~0.654) 0.011* 1.68 0.563 0.629 0.192 GLU 0.594(0.529~0.66) 0.006* 5.14 0.469 0.686 0.155 HDL 0.602(0.535~0.669) 0.003* 1.31 0.8 0.379 0.179 PT 0.6(0.536~0.664) 0.004* 12.65 0.533 0.637 0.17 TT 0.623(0.557~0.669) <0.001* 16.95 0.869 0.284 0.153 FIB 0.628(0.564~0.662) <0.001* 3.83 0.517 0.696 0.213 CEA 0.586(0.522~0.65) 0.014* 3.43 0.445 0.718 0.163 CA-199 0.569(0.504,0.635) 0.046* 7.68 0.656 0.447 0.103 CA-125 0.682(0.622,0.744) <0.0001* 14.4 0.542 0.777 0.319 *P < 0.05。 表 5 联合指标对GC转移的诊断效能
Table 5. The diagnostic effectiveness of combined clinical indexes for GC metastasis
变量 AUC(95%CI) P 最佳截断值 灵敏度 特异度 约登指数 浸润深度+PA 0.731(0.668~0.793) <0.0001* 0.303 0.775 0.588 0.363 浸润深度+CA-125 0.76(0.703~0.817) <0.0001* 0.273 0.729 0.667 0.396 PA+CA-125 0.71(0.652~0.769) <0.0001* 0.347 0.623 0.738 0.361 浸润深度+PA +CA-125 0.764(0.706~0.822) <0.0001* 0.257 0.591 0.802 0.393 *P < 0.05。 表 6 GC转移患者OS单因素及多因素分析(1)
Table 6. Univariate and multivariate analysis of OS in patients with GC metastasis (1)
变量 单因素 多因素 中位OS(月) P HR 95%CI P 性别 0.838 男 26 女 32 年龄(岁) 0.083 <45 41 ≥45 26 KPS评分 0.348 <90 28 ≥90 18.56 BMI(kg/m2) 0.612 <24 27 ≥24 26 NRS评分 0.425 <3 28 ≥3 26 病理类型 0.925 腺癌 26 其他 32 浸润深度 0.064 ≤黏膜层 44 >黏膜层 27 分化程度 0.23 高-中分化 31 其他 27 是否合并糖代谢紊乱 0.935 是 33 否 26 原发灶是否接受手术 <0.0001* 0.083 是 44 1 否 9 1.559 0.944~2.574 是否化疗 0.033* - 是 30 否 26 总化疗周期 0.043* <0.001* <8周 24 1 ≥8周 42 0.269 0.129~0.563 HER2 0.424 阴性 32 阳性 24 Epstein-Barr病毒感染 0.499 是 30 否 29 幽门螺旋杆菌感染 0.937 是 30 否 28 初诊合并转移 0.149 是 28 否 21 合并远处转移 <0.0001* 0.225 是 22 2.419 0.58~10.092 否 44 1 是否新发转移 <0.0001* 0.324 是 17 1 否 45 1.54 0.653~3.633 转移灶是否治疗 0.006* 0.52 是 20 1 否 6 1.194 0.696~2.046 *P < 0.05。 表 6 GC转移患者OS单因素及多因素分析(2)
Table 6. Univariate and multivariate analysis of OS in patients with GC metastasis (2)
变量 单因素 多因素 中位OS(月) P HR 95%CI P 区域淋巴结转移 0.287 是 27 否 44 LYMP(109/L) 0.929 ≤1.82 29 >1.82 26 NWR 0.525 <0.61 32 ≥0.61 27 NLR 0.716 <1.77 26 ≥1.77 27 LWR 0.036* - ≤0.35 15 >0.35 35 PLR 0.059 <142.26 28 ≥142.26 26 HB(g/L) 0.378 ≤145.5 20 >145.5 24 TP(g/L) 0.499 ≤65.5 26 >65.5 27 PA(g/L) 0.168 ≤216.9 22 >216.9 33 ALB(g/L) 0.007* 0.962 ≤45.5 23 1 >45.5 45 0.986 0.541~1.797 AGR 0.297 ≤1.68 23 >1.68 33 GLU(mmol/L) 0.573 <5.14 26 ≥5.14 32 HDL(mmol/L) 0.418 ≤1.31 26 >1.31 41 PT(S) 0.812 <12.65 29 ≥12.65 26 TT,S 0.762 ≤16.95 27 >16.95 21 FIB(g/L) 0.006* 0.28 <3.83 41 1 ≥3.83 20 1.314 0.8~2.158 CEA(μg/L) 0.001* 0.232 <3.63 41 1 ≥3.63 20 1.363 0.82~2.265 CA-199(KU/L) 0.179 <7.68 33 ≥7.68 23 CA-125(KU/L) <0.0001* 0.042* <14.4 44 1 ≥14.4 17 1.733 1.021~2.942 *P < 0.05。 表 7 GC转移患者PFS单因素及多因素分析(1)
Table 7. Univariate and multivariate analysis of PFS in patients with GC metastasis (1)
变量 单因素 多因素 中位PFS(月) P HR 95%CI P 性别 0.519 男 16 女 12 年龄(岁) 0.375 <45 19 ≥45 14 KPS评分 0.326 <90 16 ≥90 11 BMI 0.153 <24 17 ≥24 12 NRS评分 0.38 <3 16 ≥3 11 病理类型 0.884 腺癌 14 其他 18 浸润深度 0.151 ≤黏膜层 28 >黏膜层 22 分化程度 0.127 高-中分化 27 其他 21 是否合并糖代谢紊乱 0.565 是 10 否 16 原发灶是否接受手术 <0.0001* 0.001* 是 36 1 否 5 2.399 1.458~3.948 是否化疗 0.123 是 16 否 8 总化疗周期 0.767 <8周 16 ≥8周 17 HER2 0.072 阴性 18 阳性 16 Epstein-Barr病毒感染 0.699 是 33 否 19 幽门螺旋杆菌感染 0.727 是 18 否 18 初诊合并转移 0.001* 0.314 是 8 0.439 0.089~2.178 否 17 1 合并远处转移 <0.0001* <0.0001* 是 17 7.79 2.821~21.508 否 38 1 转移灶是否治疗 0.054 是 6 否 4 区域淋巴结转移 <0.0001* 0.016* 是 6 6.416 1.418~29.134 否 33 1 *P < 0.05。 表 7 GC转移患者PFS单因素及多因素分析(2)
Table 7. Univariate and multivariate analysis of PFS in patients with GC metastasis (2)
变量 单因素 多因素 中位PFS(月) P HR 95%CI P LYMP(109/L) 0.331 ≤1.82 17 >1.82 14 NWR 0.205 <0.61 18 ≥0.61 12 NLR 0.422 <1.77 16 ≥1.77 13 LWR 0.094 - ≤0.35 32.8 >0.35 21.71 PLR 0.101 <142.26 18 ≥142.26 12 HB(g/L) 0.462 ≤145.5 17 >145.5 13 TP(g/L) 0.428 ≤65.5 12 >65.5 16 PA(g/L) 0.436 ≤216.9 16 >216.9 16 ALB(g/L) 0.025* 0.206 ≤45.5 12 1 >45.5 36 0.714 0.424~1.204 AGR 0.717 ≤1.68 16 >1.68 16 GLU(mmol/L) 0.846 <5.14 16 ≥5.14 16 HDL(mmol/L) 0.74 ≤1.31 16 >1.31 16 PT(s) 0.74 <12.65 16 ≥12.65 16 TT(s) 0.745 ≤16.95 16 >16.95 17 FIB(g/L) 0.003* 0.103 <3.83 27 1 ≥3.83 8 1.453 0.927~2.277 CEA(μg/L) 0.001* 0.275 <3.63 32 1 ≥3.63 9 1.283 0.82~2.006 CA-199(KU/L) 0.011* 0.472 <7.68 27 1 ≥7.68 19 1.192 0.739-1.922 CA-1259(KU/L) <0.0001* 0.035* <14.4 29.81 1 ≥14.4 15.66 1.661 1.035~2.667 *P < 0.05。 -
[1] Joshi S S,Badgwell B D. Current treatment and recent progress in gastric cancer[J]. CA Cancer J Clin,2021,71(3):264-279. doi: 10.3322/caac.21657 [2] Johnston F M,Beckman M. Updates on management of gastric cancer[J]. Curr Oncol Rep,2019,21(8):67. doi: 10.1007/s11912-019-0820-4 [3] Lin M R,Yu L Z,Fu J J. Current treatment status and trends of early gastric cancer in China: analyzed based on the data of China Gastrointestinal Cancer Surgery Union[J]. Chinese Journal of Practical Surgery,2019,39(5):419-423. [4] Smyth E C,Nilsson M,Grabsch H I,et al. Gastric cancer[J]. Lancet,2020,396(10251):635-648. doi: 10.1016/S0140-6736(20)31288-5 [5] 肖正杰,田庆刚,海尔汗. 胃癌患者术后生存时间的影响因素分析[J]. 中国普外基础与临床杂志,2021,28(7):4. [6] Bray F,Ferlay J,Soerjomataram I,et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2018,68(6):394-424. doi: 10.3322/caac.21492 [7] Fan X,Qin X,Zhang Y,et al. Screening for gastric cancer in China: Advances,challenges and visions[J]. Chin J Cancer Res,2021,33(2):168-180. doi: 10.21147/j.issn.1000-9604.2021.02.05 [8] 杨之洵,郑荣寿,张思维,等. 中国胃癌发病趋势及预测[J]. 中国肿瘤,2019,28(5):321-326. [9] 高梓茗,徐惠绵. 胃癌精准外科治疗的研究新进展[J]. 中华医学信息导报,2021,36(1):7-8. doi: 10.3760/cma.j.issn.1000-8039.2021.01.106 [10] 武爱文,季加孚. 胃癌分期与临床诊治相关性[J]. 中国医学前沿杂志,2012,(5):3. [11] Youn G J,Chung W C. Micrometastasis in Gastric Cancer[J]. Korean J Gastroenterol,2017,69(5):270-277. doi: 10.4166/kjg.2017.69.5.270 [12] 姜晓东. 根治性手术与姑息性手术对老年胃癌患者的临床疗效及术后创伤的影响分析[J]. 国际医药卫生导报,2019,25(18):3. doi: 10.3760/cma.j.issn.1007-1245.2019.18.036 [13] 包永兴,惠周光. 术后放疗在接受新辅助化疗联合手术切除的非小细胞肺癌的应用进展[J]. 中华放射肿瘤学杂志,2022,31(1):7. [14] 周希山, 郭俊, 杨静, 等. 恩度与化疗联合治疗多种晚期恶性肿瘤的临床观察. 世界最新医学信息文摘, 2017, 17(5): 1. [15] Emoto S,Ishigami H,Yamashita H,Yamaguchi H,Kaisaki S,Kitayama J. Clinical significance of CA125 and CA72-4 in gastric cancer with peritoneal dissemination[J]. Gastric Cancer,2012,15(2):154-61. doi: 10.1007/s10120-011-0091-8 [16] Wang J,Wang X,Yu F,et al. Combined detection of preoperative serum CEA,CA19-9 and CA242 improve prognostic prediction of surgically treated colorectal cancer patients[J]. Int J Clin Exp Pathol,2015,8(11):14853-14863. [17] Liu X,Qiu H,Liu J,et al. Combined preoperative concentrations of CEA,CA 19-9,and 72-4 for predicting outcomes in patients with gastric cancer after curative resection[J]. Oncotarget,2016,7(23):35446-35453. doi: 10.18632/oncotarget.9060 [18] Klump K E,Mc Ginnis J F. The role of reactive oxygen species in ocular malignancy[J]. Adv Exp Med Biol,2014,801(7):655-659. [19] Lin J X,Wang Z K,Huang Y Q,et al. Dynamic Changes in Pre- and Postoperative Levels of Inflammatory Markers and Their Effects on the Prognosis of Patients with Gastric Cancer[J]. J Gastrointest Surg,2021,25(2):387-396. doi: 10.1007/s11605-020-04523-8 -






 下载:
下载: