A Comparative Study of Rat Models of Intrauterine Adhesions Constructed in Different Ways
-
摘要:
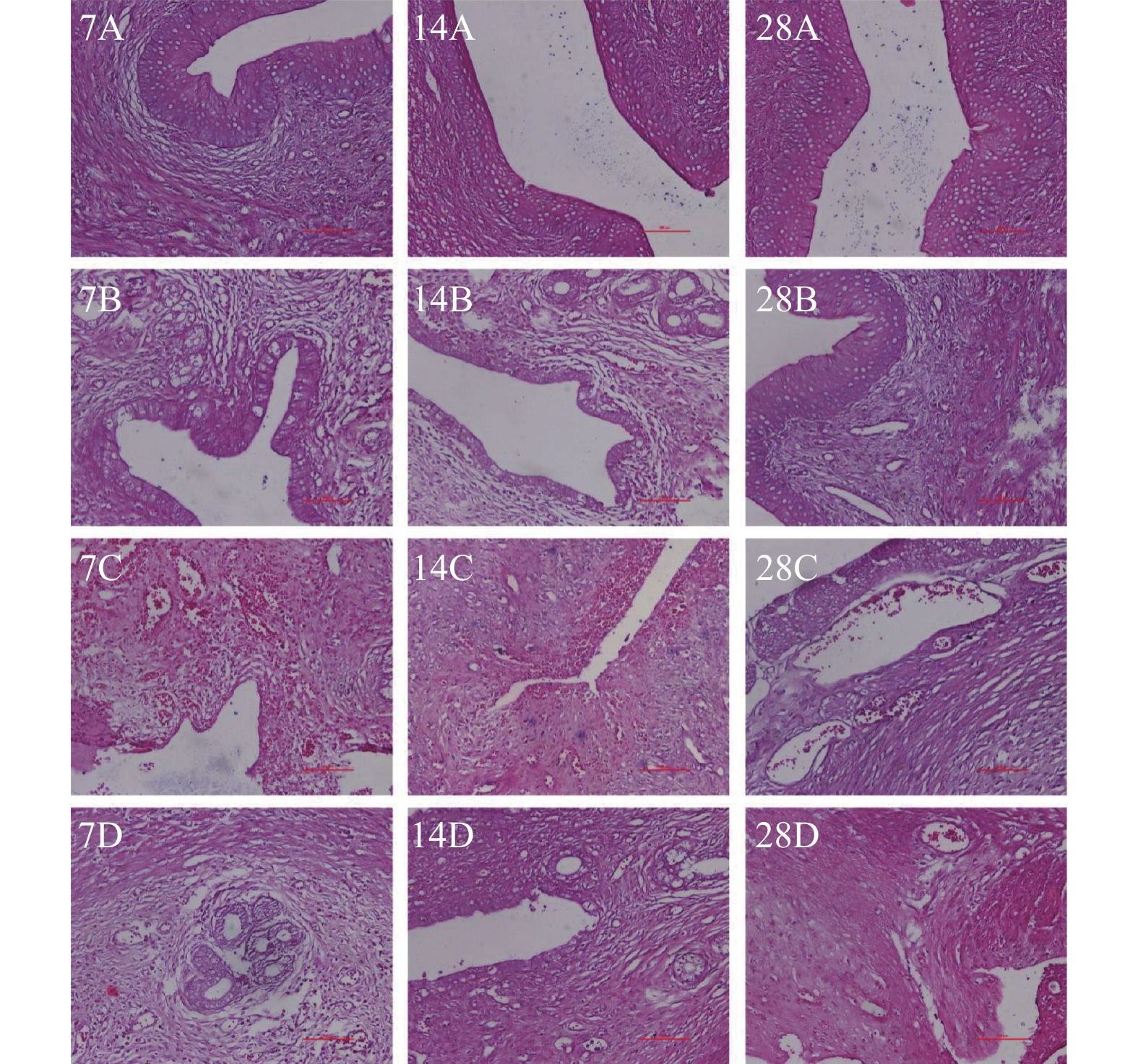
目的 通过采取不同的处理方式构建宫腔粘连大鼠模型,以筛选出更稳定的宫腔粘连大鼠模型,为进一步研究宫腔粘连的发生机制以及为新的治疗方式提供理论依据。 方法 将48只雌性发情期的SD大鼠随机分为4组,即对照组、脂多糖棉线组(带线组)、脂多糖注射组(注射组)、脂多糖明胶组(明胶组),每组12只。分别于建模7 d、14 d、28 d每组各处死4只大鼠并取子宫内膜组织检测,使用HE染色观察大鼠子宫内膜腺体数量变化情况,使用Masson染色观察大鼠子宫内膜纤维化面积变化情况,使用免疫组织化法检测纤维化蛋白TGF-β1的表达情况。使用Western Blot检测纤维化蛋白Collagen-Ⅰ、Collagen-Ⅲ、α-SMA、TGF-β1的蛋白表达情况。 结果 (1)HE染色结果显示,建模7 d、14 d、28 d,各组腺体数量均较对照组减少(P < 0.05)。其中建模14 d、28 d,注射组的腺体数量比带线组及明胶组均减少更明显(P < 0.05),且建模28 d时,注射组腺体数量几乎为0。(2)Masson染色结果显示,带线组、注射组、明胶组在建模7 d、14 d、28 d大鼠的子宫内膜纤维化程度均明显较对照组高(P < 0.05),经过不同组之间的比较发现,注射组大鼠的子宫内膜纤维化程度较带线组、明胶组高(P < 0.05),且造模28d注射组最严重。(3)免疫组化染色结果显示,在建模7 d、14 d、28 d时,带线组、注射组、明胶组等各组大鼠TGF-β1的表达均较对照组显著增加,除造模7 d的带线组、明胶组外,其余各组结果之间均具有统计学意义(P < 0.05)。建模7 d、28 d时,注射组TGF-β1的表达量较明胶组升高(P < 0.05)。(4)Western blot结果表明,机械损伤后不同的感染细菌脂多糖处理方式都会上调纤维化相关蛋白的表达,经过不同组之间的比较发现,造模7 d、14 d、28 d,注射组较明胶组Collagen-Ⅰ、Collagen-Ⅲ、α-SMA及TGF-β1表达升高(P < 0.05),但明胶组与带线组相比,组间结果无统计学意义(P > 0.05);造模7 d,注射组较带线组Collagen-Ⅰ、Collagen-Ⅲ、α-SMA蛋白表达升高(P < 0.05),造模14 d,注射组较带线组Collagen-Ⅲ、α-SMA蛋白表达升高(P < 0.05),造模28 d,注射组较带线组Collagen-Ⅰ、Collagen-Ⅲ、α-SMA及TGF-β1蛋白表达升高(P < 0.05)。且对照组相、带线组、注射组和明胶组4组中,注射组中的Collagen-Ⅰ、Collagen-Ⅲ、α-SMA及TGF-β1蛋白的表达都是最高的,经过机械损伤后,注射脂多糖损伤大鼠子宫的效果最为显著。 结论 采用刮宫后宫腔内注射脂多糖的方式所获得的大鼠宫腔粘连模型较宫腔内放置脂多糖棉线或脂多糖明胶的损伤更严重,建模效果更好,且建模28 d时的大鼠宫腔粘连模型损伤最严重。刮宫后宫腔内放置脂多糖明胶作为一种新的方法,也能造成宫腔粘连。 Abstract:Objective To find out the more stable rat models of intrauterine adhesions, so as to provide the basis for further investigation into the mechanism of intrauterine adhesions occurrence and exploration of novel treatments by constructing rat models of intrauterine adhesions with different measures. Methods Forty-eight female SD rats in estrus were randomly assigned to one of the four groups: control group, lipopolysaccharide cotton thread group (with line group), lipopolysaccharide injection group (injection group), lipopolysaccharide gelatin group (gelatin group), each group consisted of 12 rats. Rats in the gelatin group placed a gelatin sponge strip soaked in lipopolysaccharides in the uterine cavity after curettage. In each group, 4 rats were sacrificed 7 days, 14 days, and 28 days later, respectively, and endometrial tissue was detected, the number of endometrial glands and the area of fibrosis of the rats were observed by HE staining and Masson staining and the expression of the fibrotic protein TGF-β1 was detected by immunohistochemistry. Western Blot was used to detect the protein expression of fibrotic proteins Collagen-I, Collagen-III, α-SMA, and TGF-β1. Result (1) The results of HE staining showed that the number of glands in each group decreased compared with the control group after 7 days, 14 days, and 28 days of modeling (P < 0.05). Of these, after 14 days and 28 days of modeling, the number of glands in the injection group decreased more significantly than those in the line and gelatin e group (P < 0.05), and when modeling for 28 days the number of glands in the injection group was close to zero. (2) Masson staining showed that the degree of fibrosis of the endometrium in the lineage-based group, injection group, and gelatin group was significantly higher than that of the control group at 7 days, 14 days, and 28 days (P < 0.05), and after comparison between different groups, it was found that the degree of endometrial fibrosis of rats in the injection group was higher than that in the line-and-line group and gelatin group (P < 0.05), and the injection group after 28 days of molding was the most severe group. (3) Immunohistochemical staining showed that TGF-β1 expression in the lineage group, there was a significant increase in the injection group and gelatin group compared to the control group at 7 days, 14 days, and 28 days of modeling, and the differences in the other groups were statistically significant with the exception of the line and gelatin group for 7 days of molding (P < 0.05). Furthermore, at 7 days and 28 days after modeling, TGF-β1 expression in the injection group was greater than that in the gelatin group (P < 0.05). (4) Western blot analysis showed that the expression of fibrosis related proteins was up-regulated by different methods of infective bacterial lipopolysaccharide treatment after mechanical injury, and upon a comparison between different groups, it was found that the expression of Collagen-I, Collagen-III, α-SMA, and TGF-β1 in the injection group was increased in comparison to that in the gel-treated group (P < 0.05), but the difference between the gelatin e group and the strip group did not reach statistical significance (P > 0.05). After 7 days of molding, the expression Collagen-I, Collagen-III And α-SMA in the injection group was higher (P < 0.05), 14 days after molding, the expression of Collagen-III andα-SMA (P < 0.05) in the injection group was higher than that in the line group, and the expression of Collagen-I, Collagen-III, α-SMA, and TGF-β1 in the injection group was greater (P < 0.05). The expression of Collagen-I, Collagen-III, α-SMA and TGF-β1 protein in the control group, the line group, the injection group, and the gelatin group was the highest, and after mechanical injury, the effect of lipopolysaccharide injection to damage the uteri of rats was the most significant. Conclusions The rat model of intrauterine adhesions obtained by intrauterine injection of lipopolysaccharide after curettage is more severe than that obtained by intrauterine placement of a cotton string of lipopolysaccharide or lipopolysaccharide gelatin and the rat model of intrauterine adhesions has the most severe damage 28 days after modeling. The placement of lipopolysaccharide gelatin in the uterine cavity following curettage is a novel method that may also result in intrauterine adhesions. -
Key words:
- Intrauterine adhesions /
- Rats /
- Animal model /
- Modeling
-
图 4 western blot检测纤维化有关蛋白的表达情况(
$\bar x \pm s $ )与对照组相比,*P < 0.05,**P < 0.01,***P < 0.001;与带线组相比,#P < 0.05,##P < 0.01,###P < 0.001;与注射组相比,& P < 0.05,&& P < 0.01,&&& P < 0.001。A:7 d大鼠子宫组织纤维化有关蛋白检测情况;B:14 d大鼠子宫组织纤维化有关蛋白检测情况;C:21 d大鼠子宫组织纤维化有关蛋白检测。
Figure 4. The expression of fibrosis-related proteins detected by Western blot (
$\bar x \pm s $ )表 1 大鼠子宫腺体数量统计
Table 1. Number of uterine glands in rats
组别 7 d腺体
数量(个)14 d腺体
数量(个)28 d腺体
数量(个)对照组 11.00 10.00 9.50 带线组 6.25△△△ 6.00△ 5.75△ 注射组 2.00△△△ ## 1.00△△△## 0.50△△△### 明胶组 5.25△△ 5.00△△△&& 4.50△△& F 11.98 33.11 20.81 P 0.0006* < 0.001* < 0.001* *P < 0.05;与对照组比较,△P < 0.05,△△P < 0.01,△△△P < 0.001;与带线组比较,#P < 0.05,##P < 0.01;与注射组比较,&P < 0.05,&& P < 0.01。 表 2 大鼠子宫内膜纤维化程度(%)
Table 2. Area of endometrial fibrosis in rats (%)
组别 7 d纤维化程度 14 d纤维化程度 28 d纤维化程度 对照组 21.04 8.76 18.92 带线组 61.83△△ 83.89△△△ 142.99aaabbb 注射组 202.51△△△## 253.58△△△### aaa bbb 310.23△△△### aaabbb 明胶组 96.22△△△#&&& 109.54△△△&&& aaabbb 171.12△△△&&& aaabbb F 127.3 194 207.9 P < 0.001* < 0.001* < 0.001* *P < 0.05;与对照组比较,△P < 0.05,△△P < 0.01,△△△P < 0.001;与带线组比较,#P < 0.05,##P < 0.01;与注射组比较,&&& P < 0.001;与7 d比较, aaaP < 0.001;与14 d比较,bbbP < 0.001。 表 3 大鼠子宫内膜TGF-β1的蛋白阳性率比较(%)
Table 3. Comparison of protein positivity rate of endometrial TGF-β1 on the endometrium in rats (%)
组别 7 d阳性率 14 d阳性率 28 d阳性率 对照组 54.41 42.54 45.41 带线组 67.58 86.66△△△ 117.31△△△aaabb 注射组 106.45△△△ 111.15△△△ 139.87△△△aab 明胶组 80.68& 102.24△△△a 109.77△△△&& F 13.37 17.58 41.69 P 0.0018* 0.007* < 0.001* *P < 0.05;与对照组比较,△P < 0.05,△△P < 0.01,△△△P < 0.001;与带线组比较,#P < 0.05,##P < 0.01;与注射组比较,&&& P < 0.001;与7 d比较,aP < 0.01, aaaP < 0.001;与14 d比较,bP < 0.005,bbP < 0.01。 -
[1] Dreisler E,Kjer J J. Asherman's syndrome: current perspectives on diagnosis and management[J]. International journal of women's health,2019,11:191-198. doi: 10.2147/IJWH.S165474 [2] 王素敏,花向东. 不同方法对子宫内膜损伤修复的治疗结局探讨[J]. 中国实用妇科与产科杂志,2022,38(9):885-891. [3] 许阡,王祎祎,臧春逸. 宫腔粘连临床病因学及诊疗研究进展[J]. 国际妇产科学杂志,2021,48(2):224-229,240. doi: 10.12280/gjfckx.20200820 [4] 中华医学会妇产科学分会. 宫腔粘连临床诊疗中国专家共识[J]. 中华妇产科杂志,2015,50(12):881-887. doi: 10.3760/cma.j.issn.0529-567x.2015.12.001 [5] Sun L,Zhang S,Chang Q,et al. Establishment and comparison of different intrauterine adhesion modelling procedures in rats[J]. Reprod Fertil Dev,2019,38(14):149-156. [6] 梁姗姗,植枝福,黄滟岚. 宫腔粘连动物模型建立的研究进展[J]. 中华生殖与避孕杂志,2022,42(8):874-877. [7] 陈艳玲,孙冬岩. 大鼠宫腔粘连模型的研究进展[J]. 中国计划生育和妇产科,2022,14(1):49-51. [8] 付振琳,陈欣,杨菁. 藏药韦色尼阿丸预防宫腔粘连形成的实验研究[J]. 生殖医学杂志,2021,30(4):524-529. doi: 10.3969/j.issn.1004-3845.2021.04.018 [9] 江梅,姜经航,邵帅,等. 宫腔粘连大鼠模型的构建以及二甲双胍对模型大鼠生育能力的改善作用[J]. 广西医学,2021,43(1):62-66. [10] Chen Y,Chang Y,Yao S. Role of angiogenesis in endometrial repair of patients with severe intrauterine adhesion[J]. Int J Clin Exp Pathol,2013,6(7):1343-1350. [11] Kou L,Jiang X,Xiao S,et al. Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions[J]. Journal of controlled release:official journal of the Controlled Release Society,2020,318:25-37. doi: 10.1016/j.jconrel.2019.12.007 [12] 王改,贺斌,徐祥波,等. 子宫内膜损伤与宫腔粘连动物模型的研究进展[J]. 生殖医学杂志,2014,23(10):856-859. doi: 10.3969/j.issn.1004-3845.2014.10.017 [13] 黄璞,张诚,李春艳,等. 子宫内膜异位症动物模型的研究进展[J]. 现代妇产科进展,2020,29(8):634-637. doi: 10.13283/j.cnki.xdfckjz.2020.08.033 [14] 张春斌,谈西满,罗佳滨,等. 子宫内膜异位症动物模型的研究现状及进展[J]. 黑龙江医药科学,2008,31(3):80-81. doi: 10.3969/j.issn.1008-0104.2008.03.090 [15] 刘芳,何援利. 机械和感染双重损伤法建立新西兰大白兔宫腔粘连模型[J]. 重庆医学,2013,42(7):765-767. [16] 阳媛,毛艳华,王佳,等. 机械和感染双重法建立中华田园犬宫腔粘连模型[J]. 重庆医科大学学报,2017,42(4):401-405. doi: 10.13406/j.cnki.cyxb.001225 [17] 张永裕,谭国胜,罗灿桥,等. 多重损伤法建立大鼠宫腔粘连模型及其对子宫内膜LIF及整合素αvβ3的影响[J]. 中山大学学报:医学科学版,2016,37(1):15-22. [18] 施佳艳. 宫腔粘连发生的高危因素分析[J]. 蚌埠医学院学报,2021,46(3):376-377. doi: 10.13898/j.cnki.issn.1000-2200.2021.03.026 [19] 吴言英,王敏. 透明质酸钠凝胶联合明胶海绵预防宫腔粘连的研究[J]. 黑龙江医药,2017,30(6):1214-1216. doi: 10.14035/j.cnki.hljyy.2017.06.013 [20] Prianishnikov V A. On the concept of stem cell and a model of functional-morphological structure of the endometrium[J]. Contraception,1978,18(3):213-223. doi: 10.1016/S0010-7824(78)80015-8 [21] 郭意欣,关婷. 机械损伤联合不同感染方法建立大鼠宫腔粘连模型的对比研究[J]. 现代妇产科进展,2018,27(9):693-695. doi: 10.13283/j.cnki.xdfckjz.2018.09.014 [22] 曹承华,贺雅静,高荧苒,等. LPS介导的炎症反应过程及作用机制[J]. 河南大学学报:医学版,2017,36(1):70-76. [23] Xiao L,Song Y,Huang W,et al. Expression of SOX2,NANOG and OCT4 in a mouse model of lipopolysaccharide-induced acute uterine injury and intrauterine adhesions[J]. Reproductive Biology and Endocrinology,2017,15(1):14-20. doi: 10.1186/s12958-017-0234-9 [24] Dd B,Se G. Direct effects of endotoxinon the endothelium: barrier function and injury[J]. Lab Invest,1999,79(10):1181-1199. [25] Bai X,Liu J,Cao S,et al. Mechanisms of endometrial fibrosis and the potential application of stem cell therapy[J]. Discovery medicine,2019,27(150):267-279. [26] 谢小惠. 宫腔粘连患者子宫内膜TGF-β1、β-catenin的表达及雌激素与生长激素治疗的比较 [D]. 长沙: 中南大学, 2014. [27] 周柳. TGF-β1诱导的Postn、α-SMA和CollagenⅠ在宫腔粘连内膜组织中表达上调且与粘连程度相关 [D]. 重庆: 重庆医科大学, 2018. -






 下载:
下载: