Analysis on the Application of Optical Coherence Tomographic and Quantitative Flow Ratio in Intermediate In-stent Restenosis Lesions
-
摘要:
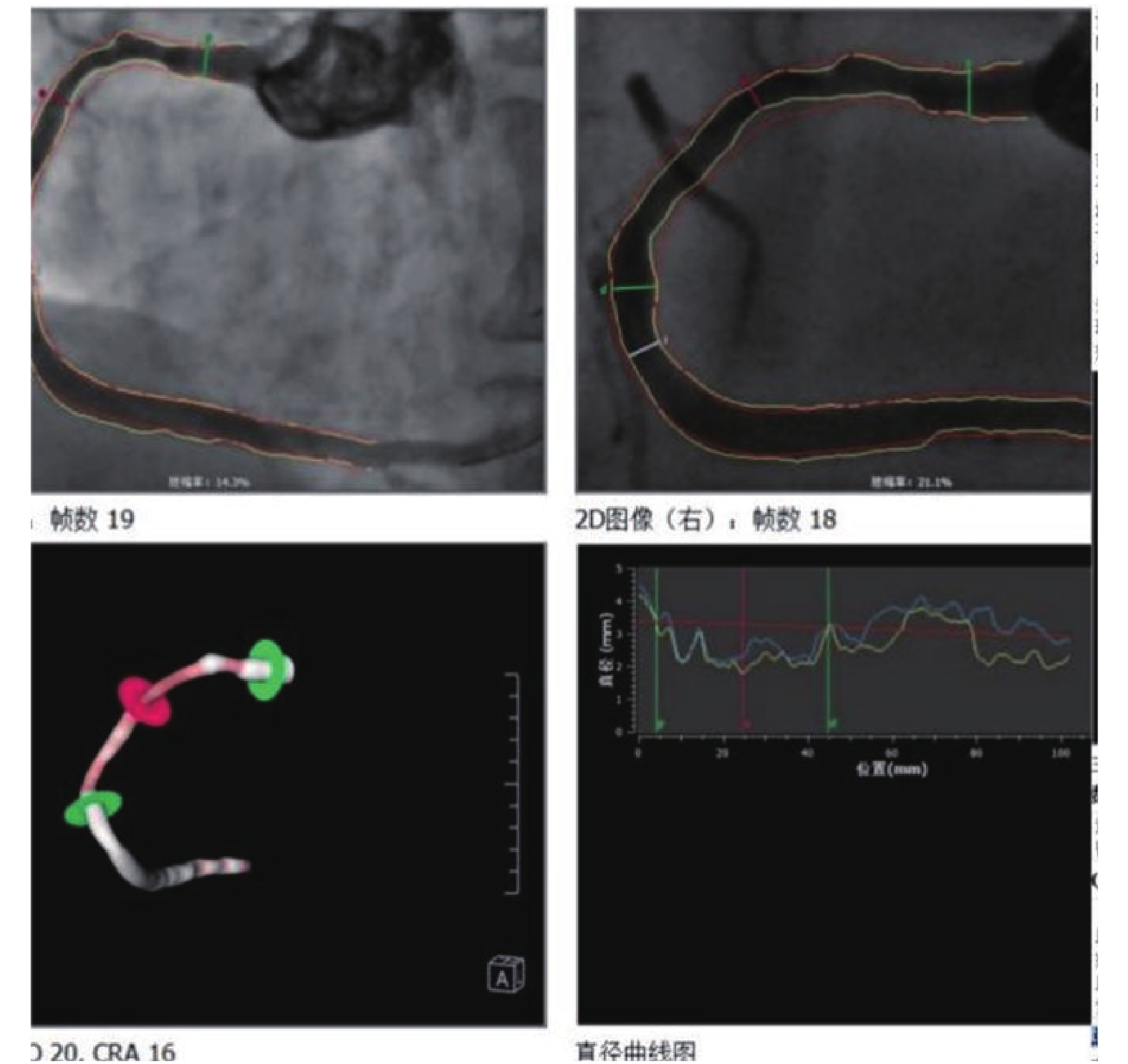
目的 观察光学相干断层成像( optical coherence tomography,OCT) 联合定量血流分数(quantitative flow ratio,QFR)对支架内再狭窄(in stent restenosis,ISR)临界病变中的指导作用。 方法 将云南省阜外心血管病医院2019年1月至2020年6月收治共48例患者,57处ISR临界病变通过OCT联合QFR指导治疗进行回顾性研究分析,讨论其在ISR临界病变中指导治疗方式的应用价值,对QFR≤0.80 或 QFR > 0.8但OCT为阳性结果的患者进行冠脉介入治疗,对QFR > 0.8且OCT为阴性结果的患者予以优化药物治疗。 结果 57处病变中29处目标血管QFR结果判断为阴性,OCT也判断阴性,继续药物保守治疗。16处目标血管QFR结果判断为阳性,OCT结果也判断阳性,分别接受了再次植入支架或药物球囊的处理。8处的目标血管QFR结果判断为阴性,而OCT结果判断阳性,分别予以药物球囊、单纯球囊扩张或支架处理。4处目标血管QFR结果判断为阳性,OCT结果为低危临界病变,均予以药物球囊的处理。随访1a的心血管主要终点事件。总体主要心血管事件(major adverse cardiac event,MACE)事件发生率较低(5.3%),各治疗组MACE事件发生率比较差异无统计学意义(P > 0.05)。 结论 OCT联合QFR治疗ISR临界病变具有重要指导意义,方便可行,能够减少不必要的手术,且能优化PCI治疗,改善临床预后。 Abstract:Objective To observe the effect of combined guidance of optical coherence tomographic (OCT) and quantitative flow ratio (QFR) in patients with In-stent restenosis (ISR) of intermediate severity. Methods Patients with intermediate ISR lesions were performed PCI or given OMT under the guidance combined OCT imaging and QFR. PCI was performed if QFR was ≤0.80 or QFR > 0.8 but OCT showed minimal luminal area < 2.0 mm2 and the in-stent plaque was unstable and more apt to rupture. Patients with QFR > 0.8 and negative OCT results were given optimal medical therapies, and major adverse cardiac events were assessed at the end of follow-up. The pre-defined primary endpoint was the composite of major adverse cardiac events or recurrent angina at one year. Results A total of 48 patients (with 57 intermediate ISR lesions ) were enrolled. There are 29 lesions of QFR (-)OCT (-); 16 lesions of QFR (+)OCT (+), 8 lesions of QFR (-)OCT (+) and 4 lesions of QFR (+)OCT (-). We found low occurrence (5.3%) of the primary endpoint of major adverse cardiac events or recurrent angina after one year follow-up.No significant difference was found in the incidence of major adverse cardiac events between the 4 groups. Conclusions In patients with intermediate ISR lesions, Combined OCT and QFR Guidance can provide useful prognostic information, reduce the number of unnecessary PCI Procedures and is associated with a lower occurrence of the composite of major adverse cardiac events -
表 1 4组患者临床基线情况比较[
$\bar x \pm s $ /n]Table 1. Baseline clinical characteristics of the patients [
$\bar x \pm s $ /n]临床资料 QFR(−)OCT(−) QFR(+)OCT(+ ) QFR(−)OCT(+) QFR(+)OCT(−) χ2 /t P 年龄(岁) 63.5 ± 7.4 61.2 ± 8.3 62.0 ± 9.5 60.8 ± 10.2 0.340 0.790 性别(男/女) 18/11 9/7 5/3 2/2 0.329 0.794 吸烟 13 5 2 1 1.733 0.682 BMI(kg/m2) 24.2 ± 3.5 23.7 ± 4.2 22.8 ± 4.5 23.5 ± 3.6 0.295 0.829 LDL-C(mmol/L) 1.74 ± 0.72 1.87 ± 0.84 2.21 ± 0.79 1.75 ± 1.15 0.756 0.524 高血压 20 13 5 2 1.961 0.509 靶血管
右冠
前降支
回旋支
12
9
8
6
7
3
2
5
1
2
2
04.064 0.836 支架植入年限(a) 1.1 ± 0.5 1.5 ± 0.7 0.9 ± 0.8 1.2 ± 0.7 2.128 0.108 表 2 不同检查组随访12月MACE事件统计(n)
Table 2. Adverse clinical events in the 3 groups at 1 year following the procedure(n)
分组 病变个数 复发心绞痛 心肌梗死 靶血管重建 心源性死亡 合计[n(%)] QFR(−)OCT(−) 29 2 0 0 0 2(6.9) QFR(+)OCT(+) 16 1 1 1 0 3(18.0) QFR(−)OCT(+) 8 0 0 1 0 1(12.5) QFR(+)OCT(−) 4 0 0 0 0 0(0.0) -
[1] Alraies M C,Darmoch F,Tummala R,et al. Diagnosis and management challenges of in-stent restenosis in coronary arteries[J]. World Journal of Cardiology,2017,9(8):640. doi: 10.4330/wjc.v9.i8.640 [2] Mcinerney A,Travieso Gonzalez A,Castro Mejia A,et al. Long‐term outcomes after deferral of revascularization of in‐stent restenosis using fractional flow reserve[J]. Catheterization and Cardiovascular Interventions,2022,99(3):723-729. doi: 10.1002/ccd.29823 [3] Vassilev D,Hazan M,Dean L. Aneurysm formation after drug‐eluting balloon treatment of drug‐eluting in‐stent restenosis: First case report[J]. Catheterization and Cardiovascular Interventions,2012,80(7):1223-1226. doi: 10.1002/ccd.23460 [4] Kang S,Mintz G S,Park D,et al. Mechanisms of in-stent restenosis after drug-eluting stent implantation: intravascular ultrasound analysis[J]. Circulation:Cardiovascular Interventions,2011,4(1):9-14. doi: 10.1161/CIRCINTERVENTIONS.110.940320 [5] Burzotta F,Leone A M,Aurigemma C,et al. Fractional flow reserve or optical coherence tomography to guide management of angiographically intermediate coronary stenosis: a single-center trial[J]. Cardiovascular Interventions,2020,13(1):49-58. doi: 10.1016/j.jcin.2019.09.034 [6] Krüger S,Koch K,Kaumanns I,et al. Clinical significance of fractional flow reserve for evaluation of functional lesion severity in stent restenosis and native coronary arteries[J]. Chest,2005,128(3):1645-1649. doi: 10.1378/chest.128.3.1645 [7] Andreini D,Mushtaq S,Pontone G,et al. CT perfusion versus coronary CT angiography in patients with suspected in-stent restenosis or CAD progression[J]. Cardiovascular Imaging,2020,13(3):732-742. [8] Cai X,Tian F,Jing J,et al. Prognostic value of quantitative flow ratio measured immediately after drug-coated balloon angioplasty for in-stent restenosis[J]. Catheterization and Cardiovascular Interventions,2021,97(S2):1048-1054. doi: 10.1002/ccd.29640 [9] Kołtowski Ł,Zaleska M,Maksym J,et al. Quantitative flow ratio derived from diagnostic coronary angiography in assessment of patients with intermediate coronary stenosis: a wire-free fractional flow reserve study[J]. Clinical Research in Cardiology,2018,107(9):858-867. doi: 10.1007/s00392-018-1258-7 [10] Lopez-Palop R,Pinar E,Lozano I,et al. Utility of the fractional flow reserve in the evaluation of angiographically moderate in-stent restenosis[J]. European Heart Journal,2004,25(22):2040-2047. doi: 10.1016/j.ehj.2004.07.016 [11] Wienemann H,Ameskamp C,Mejía-Rentería H,et al. Diagnostic performance of quantitative flow ratio versus fractional flow reserve and resting full-cycle ratio in intermediate coronary lesions[J]. International Journal of Cardiology,2022,362:59-67. [12] Kedhi E,Berta B,Roleder T,et al. TCT-416 Rationale and design of COMBINE (OCT–FFR) assessment of non-culprit lesions to better predict adverse event outcomes in diabetes mellitus patients.[J]. Journal of the American College of Cardiology,2017,70(18S):B171. [13] Batty J A,Subba S,Luke P,et al. Intracoronary imaging in the detection of vulnerable plaques[J]. Current Cardiology Reports,2016,18(3):28. doi: 10.1007/s11886-016-0705-1 [14] Sato T,Goto S,Kishi S,et al. Predictors and outcomes of ischemia-driven target lesion revascularization in deferred lesion based on fractional flow reserve: a multi-center retrospective cohort study[J]. Cardiovascular Diagnosis and Therapy,2022,12(4):485. doi: 10.21037/cdt-21-773 [15] Tomaniak M,Ochijewicz D,Kołtowski Ł,et al. OCT-derived plaque morphology and FFR-determined hemodynamic relevance in intermediate coronary stenoses[J]. Journal of Clinical Medicine,2021,10(11):2379. doi: 10.3390/jcm10112379 [16] Burzotta F,Leone A M,De Maria G L,et al. Fractional flow reserve or optical coherence tomography guidance to revascularize intermediate coronary stenosis using angioplasty (FORZA) trial: study protocol for a randomized controlled trial[J]. Trials,2014,15(1):1-9. doi: 10.1186/1745-6215-15-1 [17] Pawlowski T,Prati F,Kulawik T,et al. Optical coherence tomography criteria for defining functional severity of intermediate lesions: a comparative study with FFR[J]. The International Journal of Cardiovascular Imaging,2013,29(8):1685-1691. doi: 10.1007/s10554-013-0283-x [18] Shiono Y,Kitabata H,Kubo T,et al. Optical coherence tomography-derived anatomical criteria for functionally significant coronary stenosis assessed by fractional flow reserve[J]. Circulation Journal,2012,76(9):2218-2225. doi: 10.1253/circj.CJ-12-0195 [19] Ramasamy A,Chen Y,Zanchin T,et al. Optical coherence tomography enables more accurate detection of functionally significant intermediate non-left main coronary artery stenoses than intravascular ultrasound: a meta-analysis of 6919 patients and 7537 lesions[J]. International Journal of Cardiology,2020,301:226-234. doi: 10.1016/j.ijcard.2019.09.067 [20] Xu B,Tu S,Qiao S,et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis[J]. Journal of the American College of Cardiology,2017,70(25):3077-3087. doi: 10.1016/j.jacc.2017.10.035 [21] Xu B,Tu S,Song L,et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre,randomised,sham-controlled trial[J]. The Lancet,2021,398(10317):2149-2159. doi: 10.1016/S0140-6736(21)02248-0 [22] Hoshino M,Yonetsu T,Kanaji Y,et al. Impact of baseline plaque characteristic on the development of neoatherosclerosis in the very late phase after stenting[J]. Journal of Cardiology,2019,74(1):67-73. doi: 10.1016/j.jjcc.2019.01.002 [23] Kedhi E,Roleder T,Hermanides R,et al. TCT CONNECT-281 clinical outcomes of optical coherence tomography detected high-risk versus low-risk coronary atherosclerotic lesions in medically treated fractional flow reserve negative lesions in diabetes mellitus patients:The COMBINE trial[J]. Journal of the American College of Cardiology,2020,76(17 Supplement S):B122. [24] Sumino Y,Yonetsu T,Ueno H,et al. Impact of neoatherosclerosis observed at very late phase after coronary stent implantation on subsequent adverse events[J]. European Heart Journal,2020,41(Supplement_2):a310-a946. [25] Matsuhiro Y,Nishino M,Nakamura H,et al. P2691 Excimer laser coronary angioplasty can achieve favorable clinical outocomes for in-stent restenosis lesion with neoatherosclerosis[J]. European Heart Journal,2019,40(Supplement_1):z748-z1009. doi: 10.1093/eurheartj/ehz748 [26] Depta J P,Patel J S,Novak E,et al. Outcomes of coronary stenoses deferred revascularization for borderline versus nonborderline fractional flow reserve values[J]. The American Journal of Cardiology,2014,113(11):1788-1793. doi: 10.1016/j.amjcard.2014.03.004 [27] Nam C,Rha S,Koo B,et al. Usefulness of coronary pressure measurement for functional evaluation of drug-eluting stent restenosis[J]. The American Journal of Cardiology,2011,107(12):1783-1786. doi: 10.1016/j.amjcard.2011.02.328 [28] Kuramitsu S,Matsuo H,Shinozaki T,et al. Two-year outcomes after deferral of revascularization based on fractional flow reserve: the J-CONFIRM Registry[J]. Circulation:Cardiovascular Interventions,2020,13(1):e8355. -






 下载:
下载: