Effects of Store-operated Calcium Channel Molecule Orai3 on Rat Coronary Artery Smooth Muscle Cells Proliferation
-
摘要:
目的 探讨钙库操纵型钙通道Orai3对冠状动脉血管平滑肌细胞(CASMC)增殖的影响。 方法 培养原代冠状动脉血管平滑肌细胞(coronary vascular smooth muscle cells,CASMC),对CASMC中Orai3分子实施干扰和过表达干预,QPCR实验和Western Blot检测Orai3表达量,CCK8实验和细胞计数检测CASMC增殖情况。 结果 CCK8实验和细胞计数实验显示无血清组较10%血清组CASMC增殖速率下降(P < 0.05),CASMC中Orai3表达量下降(P < 0.05)。干扰Orai3后与对照组相比CASMC增殖速率下降(P < 0.05),过表达Orai3后与对照组相比细胞增殖速率升高(P < 0.05)。 结论 钙库操纵型钙通道组成分子Orai3在CASMC增殖中发挥重要作用。 -
关键词:
- 冠状动脉血管平滑肌细胞 /
- 细胞增殖 /
- Orai3 /
- 钙库操纵型钙通道
Abstract:Objective To investigate the effect of calcium store-operated calcium channel Orai3 on the proliferation of coronary artery smooth muscle cells (CASMC). Methods Coronary artery smooth muscle cells (CASMC) were cultured, and Orai3 molecule in CASMC was interfered and over-expressed. QPCR and Western Blot were used to detect the expression of Orai3, and CCK8 and cell counts were used to detect the CASMC proliferation. Results CCK8 assay and cell counts showed that the proliferation rate of CASMC in serum-free group was lower than that in 10% serum group (P < 0.05), and also the expression of Orai3 in CASMC was decreased (P < 0.05). Compared with the control group, the proliferation rate of CASMC decreased after interference with Orai3 (P < 0.05), and the proliferation rate of CASMC increased compared with the control group after over-expression of Orai3 (P < 0.05). Conclusion The store-operated calcium channel constituent molecule Orai3 plays an important role in CASMC proliferation. -
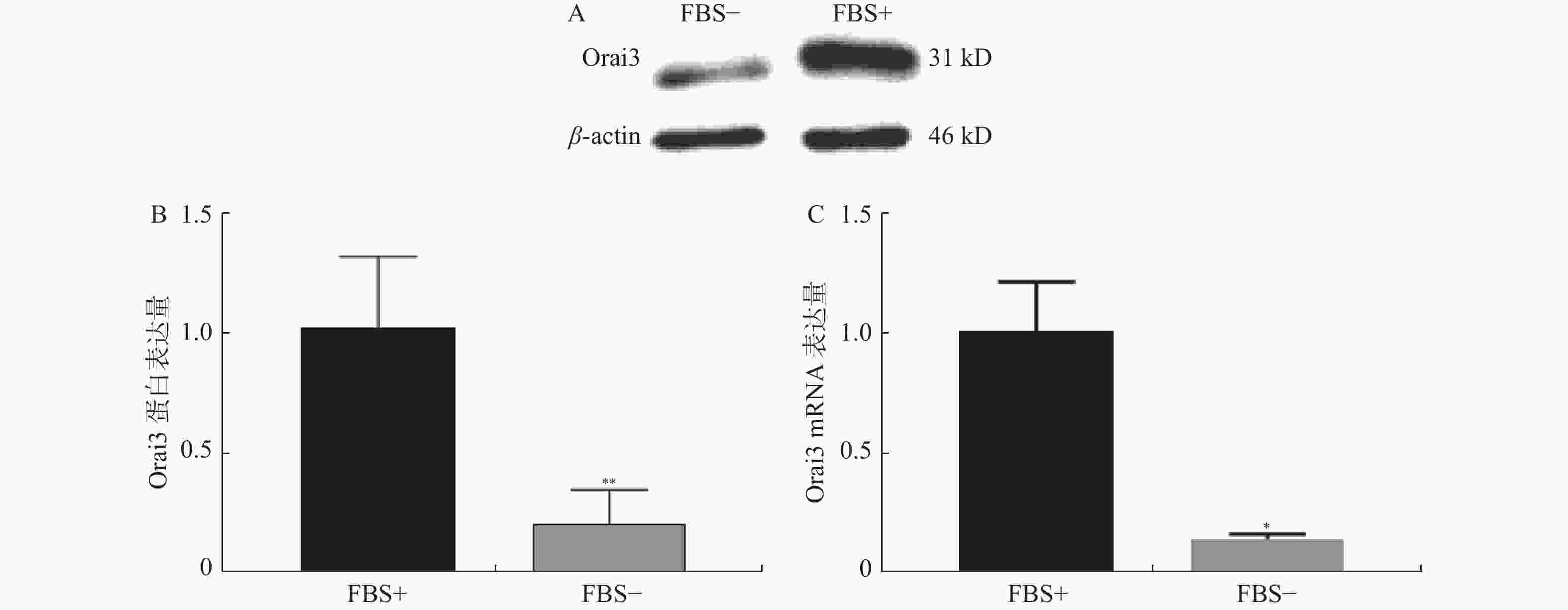
图 2 无血清组及10%血清组Orai3表达量情况
A:血清组(FBS-)和10%血清组(FBS+)培养下CASMC细胞提取蛋白Western Blot检测Orai3表达量条带图;B:Western Blot实验分别检测无血清组(FBS-)和10%血清组(FBS+)培养下CASMC增殖情况;C:QPCR实验分别检测无血清组(FBS-)和10%血清组(FBS+)培养下CASMC细胞增殖情况。与FBS+比较,*P < 0.05,**P < 0.01。
Figure 2. Orai3 expression difference between 0%FBS medium cultured group and 10% FBS medium cultured group
图 3 干扰Orai3后CASMC细胞增殖情况
A :干扰Orai3后CASMC细胞提取蛋白Western Blot检测Orai3表达量条带图。B:干扰Orai3后Western Blot实验检测干扰效率;C:干扰Orai3后QPCR实验检测干扰效率;D:干扰Orai3后CCK8实验检测CASMC增殖情况;E:干扰Orai3后细胞计数实验检测CASMC增殖情况;siOrai3-NC:Orai3干扰阴性对照组;siOrai3:Orai3干扰组。与FBS+比较,**P < 0.01。
Figure 3. Orai3 interfering and the Coronary vessel smooth muscle cell proliferation.
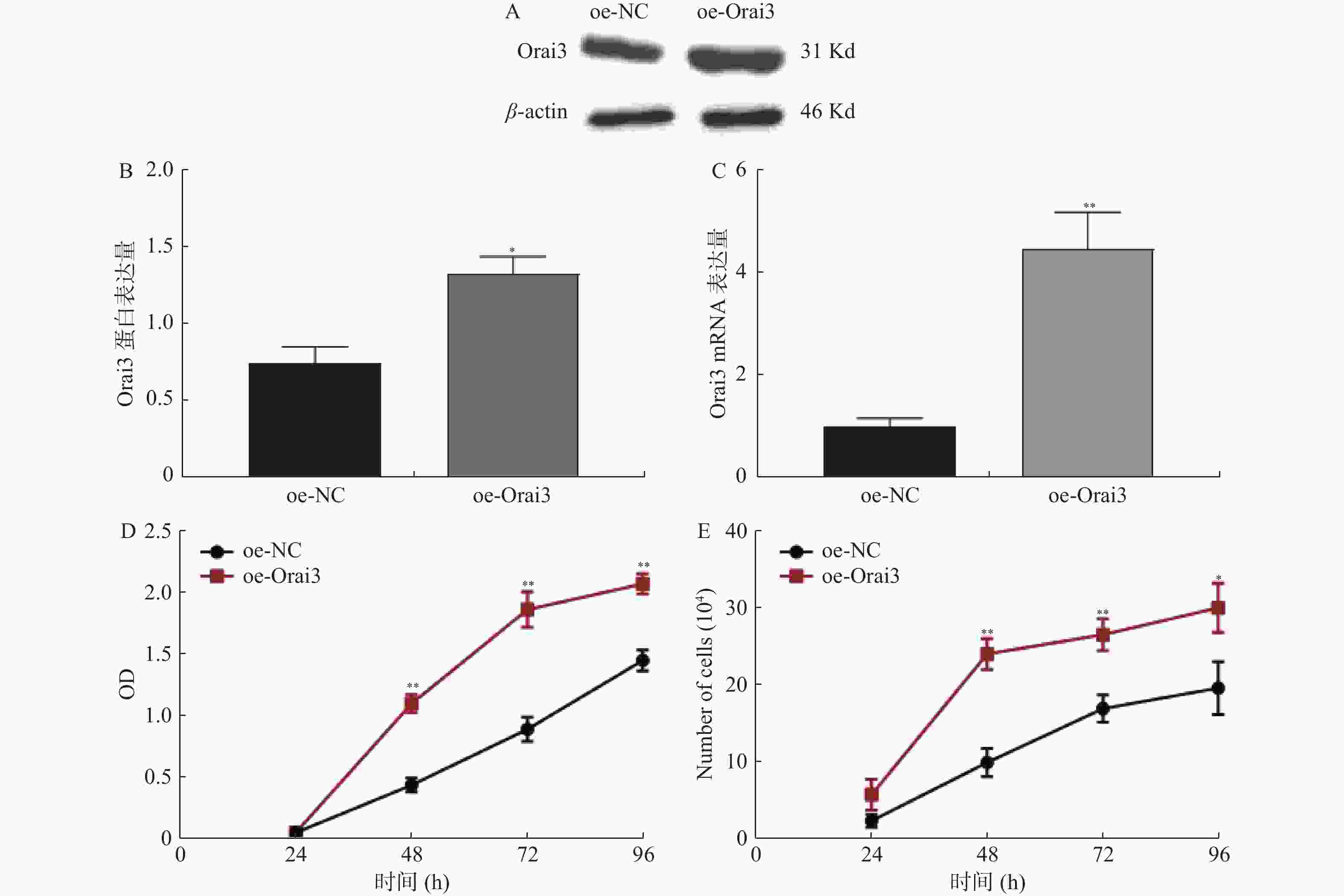
图 4 过表达Orai3后CASMC细胞增殖情况
A:过表达Orai3后CASMC细胞提取蛋白Western Blot检测Orai3表达量条带图;B:过表达Orai3后Western Blot实验检测干扰效率;C:过表达Orai3后QPCR实验检测干扰效率;D:过表达Orai3后CCK8实验检测CASMC细胞增殖情况;E:过表达Orai3后细胞计数实验检测CASMC细胞增殖情况;oe-NC:Orai3干扰阴性对照组即空白载体;oe-Orai3:Orai3干扰组。与FBS+比较,*P < 0.05,**P < 0.01。
Figure 4. Orai2 over-expression and the Coronary vessel smooth muscle cell proliferation
表 1 CCK8检测CASMC增殖[(
$\bar x \pm s $ ),n = 5]Table 1. Growth curve of CASMC cells by CCK8 assay [(
$\bar x \pm s $ ),n = 5]时间(h) FBS+ FBS− t P 0 0.0634 ± 0.0291 0.0804 ± 0.0310 0.8947 0.3971 24 0.3662 ± 0.1092 0.2629 ± 0.0645 1.8200 0.1062 48 1.0450 ± 0.0942 0.4709 ± 0.0642 11.2700 < 0.0001** 72 1.6350 ± 0.1015 0.5659 ± 0.0946 17.2300 < 0.0001** 96 1.9710 ± 0.1611 0.5015 ± 0.0905 17.7800 < 0.0001** FBS+为10%血清的完全培养液培养,FBS-为无血清的不完全培养基培养。与FBS+组比较,**P < 0.01。 表 2 CASMC细胞计数(1×104)[(
$\bar x \pm s $ ),n = 5]Table 2. Growth curve of CASMC cells by cell counting assay (1×104)[(
$\bar x \pm s $ ),n = 5]时间(h) FBS+ FBS− t P 0 1.3400 ± 0.3209 1.6000 ± 0.4183 1.1030 0.3022 24 4.9000 ± 0.7416 3.3500 ± 0.4183 4.0700 0.0036** 48 11.7000 ± 0.9747 4.4500 ± 0.9083 12.1700 < 0.0001** 72 13.5500 ± 1.0950 4.7000 ± 0.8909 14.0100 < 0.0001** 96 14.3500 ± 0.9618 3.5500 ± 1.0370 17.0800 < 0.0001 ** FBS+为10%血清的完全培养液培养,FBS-为无血清的不完全培养基培养。 与FBS+比较,**P < 0.01。 表 3 CASMC的Orai3相对表达量[(
$\bar x \pm s $ ),n = 3]Table 3. Orai3 expression of CASMC cells [(
$\bar x \pm s $ ),n = 3]Orai3 FBS+ FBS- t P 蛋白表达量 1.0270 ± 0.2925 0.2036 ± 0.1434 7.0090 0.0060** RNA表达量 1.0150 ± 0.2065 0.1498 ± 0.0167 7.5450 0.0171* FBS+为10%血清的完全培养液培养,FBS-为无血清的不完全培养基培养。与FBS+比较,*P < 0.05,**P < 0.01。 表 4 CCK8检测siOrai3干扰后CASMC增殖情况[(
$\bar x \pm s $ ),n = 5]Table 4. Growth curve of siOrai3 CASMC cells by CCK8 assay[ (
$\bar x \pm s $ ),n = 5]时间(h) siOrai3-NC siOrai3 t P 24 0.0801 ± 0.0288 0.0985 ± 0.0418 0.8098 0.4415 48 0.4464 ± 0.0603 0.1966 ± 0.0130 9.0590 < 0.0001** 72 0.8322 ± 0.1142 0.6127 ± 0.0842 3.4590 0.0086** 96 1.4200 ± 0.0547 0.6499 ± 0.1201 13.0400 0.0034** siOrai3-NC为转染siOrai3阴性对照组,siOrai3为转染siOrai3实验组。与siOrai3-NC比较,**P < 0.01。 表 5 siOrai3干扰后CASMC细胞计数(1×104)(
$\bar x \pm s $ ,n = 3)Table 5. Growth curve of siOrai3 CASMC cells by cell counting assay (1×104)(
$\bar x \pm s $ ,n = 3)时间(h) siOrai3-NC siOrai3 t P 24 2.0830 ± 1.0100 1.2500 ± 0.2500 1.3870 0.2378 48 8.1670 ± 1.2580 4.4170 ± 0.6292 4.6170 0.0099 ** 72 12.0000 ± 1.0000 7.0000 ± 0.5000 7.7460 0.0015** 96 14.0000 ± 1.0000 7.8330 ± 0.7638 8.4880 0.0011** siOrai3-NC为转染siOrai3阴性对照组,siOrai3为转染siOrai3实验组。与NC组比较,**为P < 0.01。 表 6 CCK8检测oe-Orai3转染后CASMC增殖情况[(
$\bar x \pm s $ ),n = 5]Table 6. Growth curve of oe-Orai3 CASMC cells by CCK8 assay [(
$\bar x \pm s $ ),n = 5]时间(h) oe-NC oe-Orai3 t P 24 0.0621 ± 0.0143 0.0640 ± 0.0062 0.2802 0.7865 48 0.4469 ± 0.0564 1.1080 ± 0.0712 16.2700 < 0.0001** 72 0.8993 ± 0.0979 1.8710 ± 0.1437 12.4900 < 0.0001 ** 96 1.4570 ± 0.0842 2.0770 ± 0.0820 11.7800 < 0.0001** oe-NC为转染阴性对照组,Orai2、Orai3阴性对照组为同一空白载体;oe-Orai2为过表达Orai2质粒DNA组,oe-Orai3为过表达Orai3质粒DNA组。与NC组比较,**为P < 0.01。 表 7 oe-Orai3转染后CASMC细胞计数(1×104)[(
$\bar x \pm s $ ),n = 3]Table 7. Growth curve of oe-Orai3 CASMC cells by cell counting assay[ (
$\bar x \pm s $ ),n = 3]时间(h) oe-NC oe-Orai3 t P 24 2.5000 ± 0.8660 5.9170 ± 2.0050 2.7090 0.0536 48 10.0800 ± 1.8430 24.1700 ± 2.0210 8.9190 0.0009 ** 72 17.0800 ± 1.7740 26.6700 ± 2.0820 6.0690 0.0037** 96 19.7500 ± 3.4370 30.1700 ± 3.2150 3.8340 0.0186* oe-NC为转染阴性对照组,Orai2、Orai3阴性对照组为同一空白载体;oe-Orai2为过表达Orai2质粒DNA组,oe-Orai3为过表达Orai3质粒DNA组。与NC组比较,*P < 0.05,**P < 0.01。 -
[1] Bennett M R,Sinha S,Owens G K. Vascular smooth muscle cells in atherosclerosis[J]. Circ Res,2016,118(4):692-702. doi: 10.1161/CIRCRESAHA.115.306361 [2] Guo R W,Wang H,Gao P,et al. An essential role for stromal interaction molecule 1 in neointima formation following arterial injury[J]. Cardiovasc Res,2009,81(4):660-668. doi: 10.1093/cvr/cvn338 [3] Mammadova-Bach E,Nagy M,Heemskerk J W M,et al. Store-operated calcium entry in thrombosis and thrombo-inflammation[J]. Cell Calcium,2019,77:39-48. doi: 10.1016/j.ceca.2018.11.005 [4] Fahrner M, Schindl R, Romanin C. Studies of structure-function and subunit composition of Orai/STIM channel[M]. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis, 2018: 25-50. [5] Tanwar J,Motiani R K. Role of SOCE architects STIM and Orai proteins in cell death[J]. Cell Calcium,2018,69:19-27. doi: 10.1016/j.ceca.2017.06.002 [6] Sanchez-Collado J,Jardin I,López J J,et al. Role of Orai3 in the pathophysiology of cancer[J]. Int J Mol Sci,2021,22(21):11426. doi: 10.3390/ijms222111426 [7] Zimmermann O,Zwaka T P,Marx N,et al. Serum starvation and growth factor receptor expression in vascular smooth muscle cells[J]. J Vasc Res,2006,43(2):157-165. doi: 10.1159/000090945 [8] Codeluppi S,Gregory E N,Kjell J,et al. Influence of rat substrain and growth conditions on the characteristics of primary cultures of adult rat spinal cord astrocytes[J]. J Neurosci Methods,2011,197(1):118-127. doi: 10.1016/j.jneumeth.2011.02.011 [9] Pinto M C,Kihara A H,Goulart V A,et al. Calcium signaling and cell proliferation[J]. Cellular signalling,2015,27(11):2139-2149. doi: 10.1016/j.cellsig.2015.08.006 [10] Shawer H,Norman K,Cheng C W,et al. ORAI1 Ca2+ channel as a therapeutic target in pathological vascular remodelling[J]. Front Cell Dev Biol,2021,9:653812. doi: 10.3389/fcell.2021.653812 [11] Sanchez-Collado J,Lopez J J,Cantonero C,et al. Orai2 modulates Store-Operated Ca2+ entry and cell cycle progression in breast cancer cells[J]. Cancers,2021,14(1):114. doi: 10.3390/cancers14010114 [12] Borowiec A S,Bidaux G,Tacine R,et al. Are Orail and Orai3 channels more important than calcium influx for cell proliferation?[J]. Biochim Biophys Acta,2014,1843(2):464-472. doi: 10.1016/j.bbamcr.2013.11.023 [13] Dubois C,Vanden Abeele F,Lehen'kyi V,et al. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cance[J]. Cancer Cell,2014,26(1):19-32. doi: 10.1016/j.ccr.2014.04.025 [14] Dubois C,Kondratska K,Kondratskyi A,et al. ORAI3 silencing alters cell proliferation and promotes mitotic catastrophe and apoptosis in pancreatic adenocarcinoma[J]. Biochim Biophys Acta Mol Cell Res,2021,1868(7):119023. doi: 10.1016/j.bbamcr.2021.119023 -






 下载:
下载: