Use and Replacement of Treatment Regimens for AIDS Patients on Antiretroviral Therapy
-
摘要:
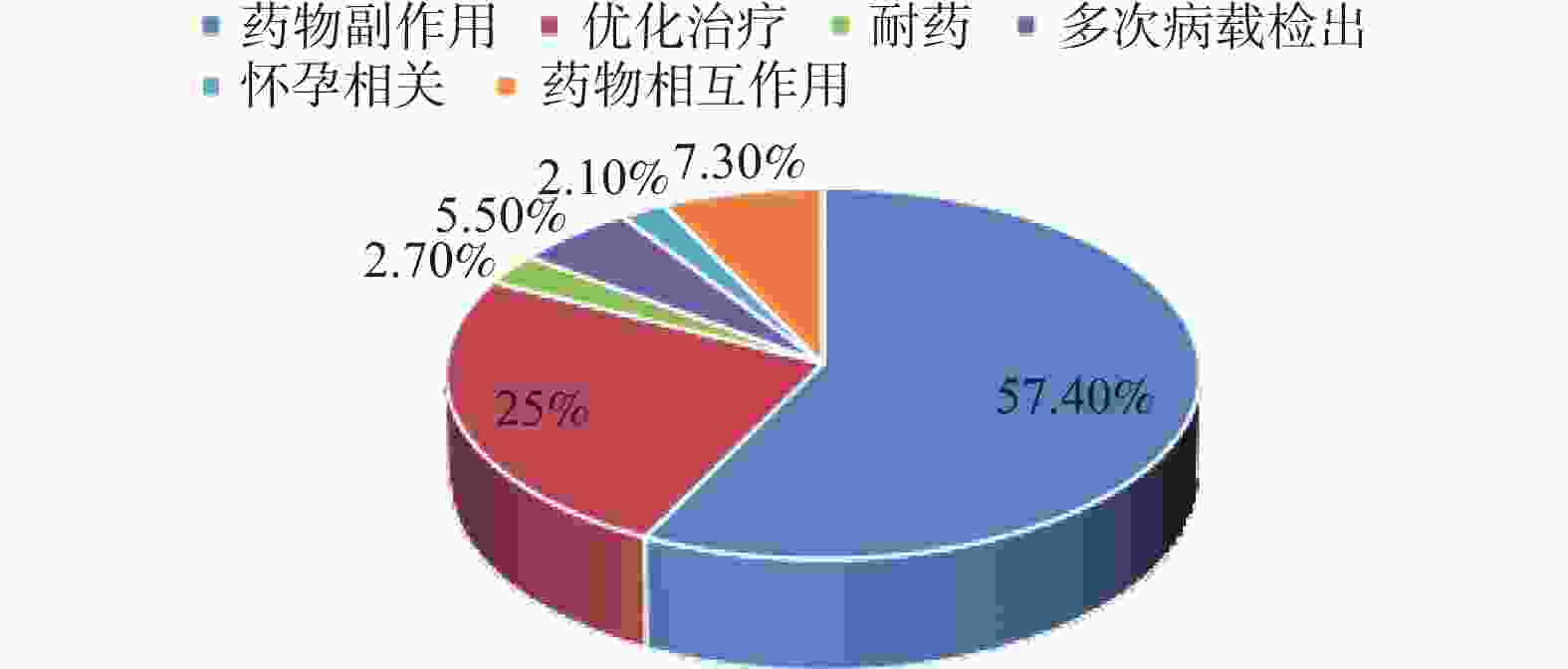
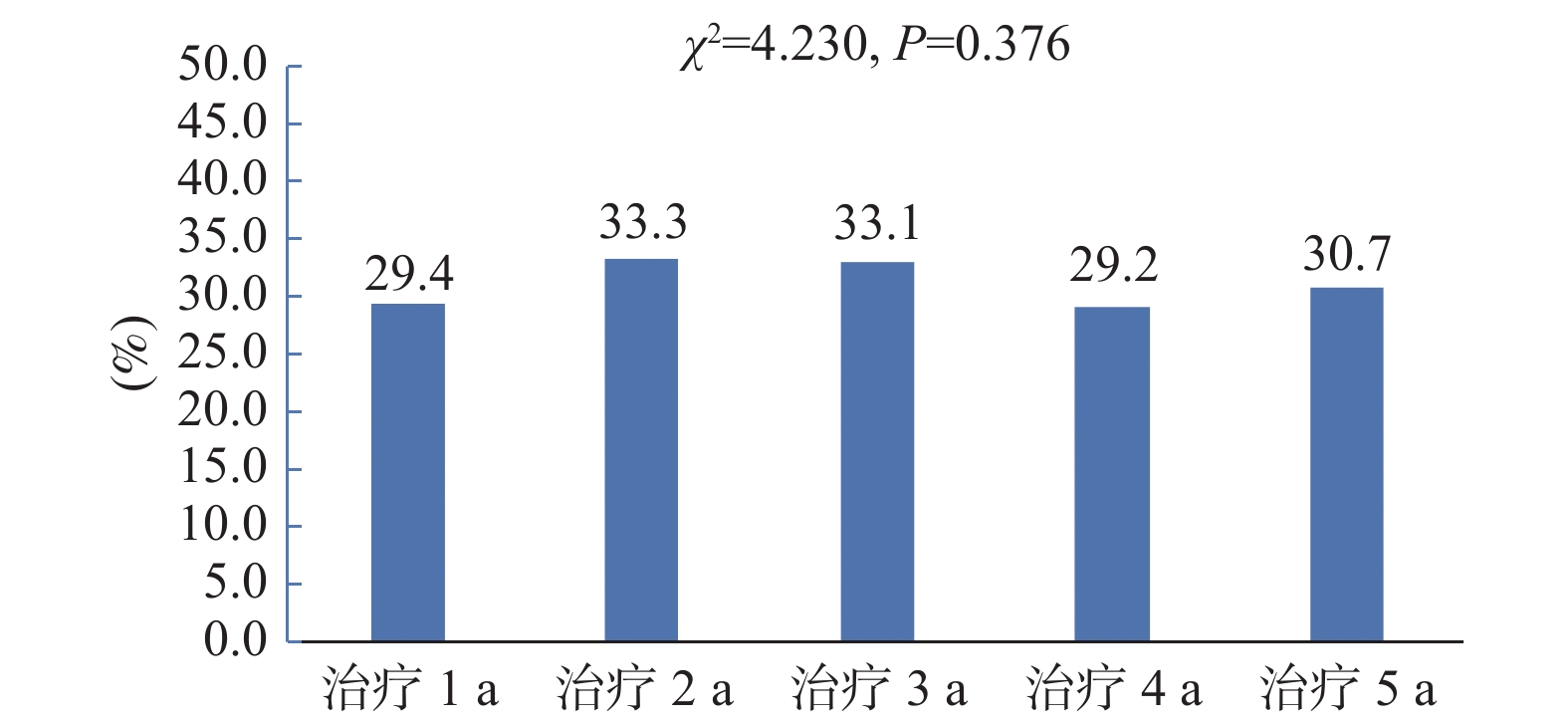
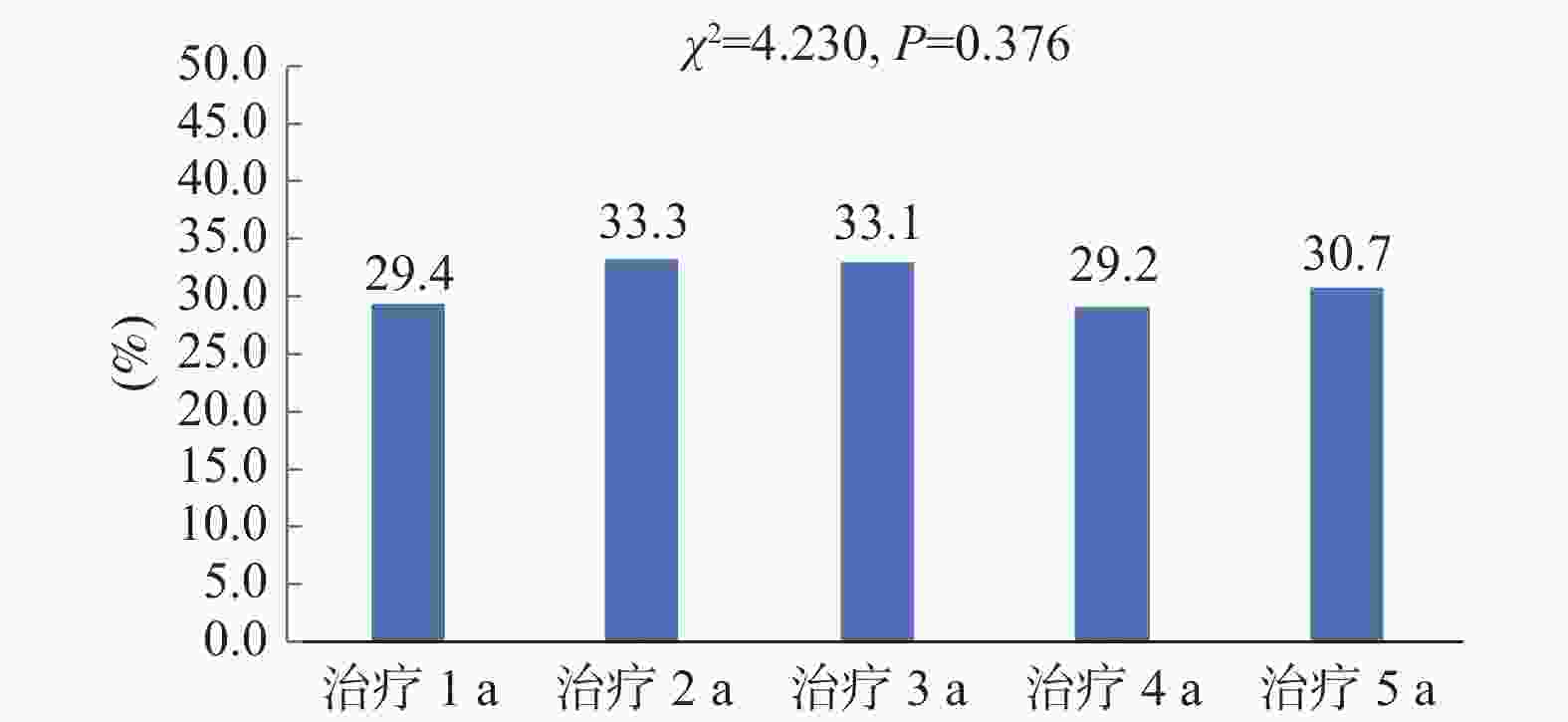
目的 了解不同时期艾滋病抗病毒治疗患者初治及经治ART方案的使用情况,分析不同药物组合的换药情况及换药原因。 方法 选取2017年1月至2022年6月初始入组ART的患者纳入研究,分析不同时期入组者人口学特征及初治与经治ART方案的变化,同时比较不同方案的换药率差异。 结果 研究对象以男性为主,平均年龄(41.35±14.2)岁,且有增大的趋势(P < 0.001)。初治患者ART方案以TDF/AZT+3TC+EFV为主,而治疗后使用率有所下降。换药率以TDF+3TC+EFV方案最高(17.3%);含LPV/r的方案使用率与初治时相比有增加;含整合酶的方案在2021年和2022年初治患者中增加明显(P < 0.001)。换药率在治疗1 a、2 a、3 a、4 a、5 a间没有差异(P = 0.376)。换药原因主要为药物副作用(57.4%)。 结论 ART药物的选择由核苷非核苷类逐渐向整合酶抑制剂转换,更趋向于个体化,结合患者的临床特点及合并症选择最适合患者的高效、低毒、简便的方案更有利于患者长期有效的治疗。 Abstract:Objective To understand the use of primary and menstrual ART regimens for HIV patients receiving antiviral treatment in different periods, and to analyze the dressing change situation and reasons of different drug combinations. Methods Patients initially enrolled in ART from January 2017 to June 2022 were included in the study. The demographic characteristics and the changes of the primary and menstrual ART regimens of the participants in different periods were analyzed and the differences in dressing change rates between the different regimens were compared. Results Most of the subjects were male, The average age was 41.35±14.2 years old and there was an increasing trend (P < 0.001). TDF/AZT+3TC+EFV was the main ART regimens in newly diagnosed patients, but the utilization rate decreased after the treatment. The TDF+3TC+EFV regimen had the highest dressing change rate (17.3%). Compared with the initial treatment, the utilization rate of the protocol containing LPV/r increased. Integrase-containing regimens increased significantly in the treated patients in 2021 and early 2022 (P < 0.001). Drug change rates did not differ at 1, 2, 3, 4, and 5 years of treatment (P = 0.376). The main reason for dressing change was drug side effects (57.4%). Conclusion The selection of ART drugs gradually switches from nucleoside and non-nucleosides to integrase inhibitors.The choice of ART drugs is gradually changing from the nucleoside non-nucleoside to integrase inhibitor, which tends to be individualized. Combining the clinical characteristics and complications of patients and choosing the most efficient, low-toxic and simple scheme are more conducive to the long-term effective treatment of patients. -
Key words:
- HIV /
- AIDS /
- ART /
- Programme /
- Change of dressing
-
表 1 2017~2022年ART初治患者人口学特征及初治方案使用情况[
$ \bar x \pm s $ /M(P25,P75)/ n(%)]Table 1. Demographic characteristics and use of initial treatment regiments of ART patients from 2017 to 2022[
$ \bar x \pm s $ /M(P25,P75)/ n(%)]项目 2017年(n = 752) 2018年(n = 743) 2019年(n = 574) 2020年(n = 528) 2021年(n = 476) 2022年(n = 226) F/χ2 P 治疗时年龄(岁)
性别 男
婚姻状况
未婚
已婚
离异丧偶
传播途径
异性
同性
静脉吸毒
其他
存活在治
初始CD4 (/µL)
初始治疗方案
TDF+3TC+EFV
AZT+3TC+EFV
AZT+3TC+LPV/r
TDF+3TC+LPV/r
AZT+3TC+NVP
20含整合酶方案38.9±12.6
527(70.1)
251(33.4)
340(45.2)
161(21.4)
477(63.4)
163(21.7)
63(8.4)
49(6.5)
636(84.6)
246(125,387)
614(81.6)
95(12.6)
21(2.8)
9(1.2)
10(1.3)
0(0.0)40.2±14.5
492(66.2)
271(36.5)
350(47.1)
122(16.4)
453(61.0)
150(20.2)
67(9.0)
73(9.8)
617(83.0)
233(98,370)
562(75.6)
128(17.2)
21(2.8)
11(1.5)
6(0.8)
14(1.88)41.3±13.8
388(67.6)
203(35.4)
265(26.1)
106(18.5)
368(64.1)
89(15.5)
43(7.5)
74(12.9)
459(80.0)
225(101,360)
370(64.5)
87(15.2)
22(3.8)
11(1.9)
2(0.3)
80(13.94)42.4±14.5
371(70.3)
193(36.6)
249(47.1)
86(16.3)
313(59.3)
83(15.7)
55(10.4)
77(14.6)
435(82.4)
219(95,363)
305(57.8)
69(13.1)
7(1.3)
5(0.9)
4(0.8)
135(25.57)44.0±15.1
340(71.4)
164(34.5)
214(44.9)
98(20.6)
330(69.3)
66(14.0)
33(6.9)
47(9.8)
400(84.0)
213(92,357)
156(23.8)
78(16.4)
11(2.3)
6(1.3)
0(0.0)
225(47.27)45.4±14.6
149(65.9)
76(33.6)
103(45.6)
47(20.8)
183(81.0)
36(15.9)
6(2.7)
1(0.4)
-
220(98,341)
49(21.7)
22(9.7)
6(2.7)
4(1.8)
0(0.0)
132(58.41)56.721
6.178
0.189
-
5.510
8.376
-< 0.001*
0.289
0.424
< 0.001*
0.239
0.137
< 0.001**P < 0.05。 表 2 各年入组在治患者当前ART方案使用情况[n(%)]
Table 2. Current use of ART regimen in the enrolled patients in each year [n(%)]
项目 2017年
(n = 636)2018年
(n = 617)2019年
(n = 459)2020年
(n = 435)2021年
(n = 400)2022年
(n = 208)TDF+3TC+EFV
AZT+3TC+EFV
AZT+3TC+LPV/r
TDF+3TC+LPV/r
AZT+3TC+NVP
DTG+两核苷
含RAL
含ABC
DTG+3TC
LPV/r+3TC
BIC/FTC/TAF
EVG/c/FTC/TAF369(58.0)
59(9.2)
43(6.7)
47(7.3)
1(0.1)
13(2.0)
0(0.0)
14(2.2)
12(1.8)
9(1.4)
37(5.8)
31(4.8)335(54.2)
70(11.3)
42(6.8)
44(7.1)
1(0.1)
13(2.1)
0(0.0)
11(1.7)
19(3.0)
9(1.4)
47(7.6)
23(3.7)213(46.4)
51(11.1)
27(5.8)
36(7.8)
1(0.2)
24(5.2)
0(0.0)
10(2.1)
19(4.1)
6(1.3)
39(8.4)
29(6.3)186(42.8)
31(7.1)
20(4.5)
25(5.7)
0(0.0)
42(9.6)
2(0.4)
12(2.7)
16(3.6)
4(0.9)
46(10.5)
48(11.0)99(24.7)
41(10.2)
12(3.0)
18(4.5)
0(0.0)
40(10.0)
0(0.0)
3(1.7)
43(0.7)
2(0.5)
68(17.0)
74(18.5)39(18.7)
17(8.1)
2(0.9)
7(3.3)
0(0.0)
26(12.5)
1(0.4)
2(0.9)
41(19.7)
3(1.4)
54(25.9)
3(1.4)表 3 不同ART方案在初治时和当前的使用率比较[n(%)]
Table 3. Comparison of current and initial use rates of different ART regimens [n(%)]
项目 初治使用率
(n = 3 299)当前使用率
(n = 2 755)换药率(%) χ2 P TDF+3TC+EFV
AZT+3TC+EFV
AZT+3TC+LPV/r
TDF+3TC+LPV/r
DTG+两核苷
含RAL
DTG+3TC
LPV/r+3TC
BIC/FTC/TAF
EVG/c/FTC/TAF2056(62.3)
479(14.5)
88(2.7)
46(1.4)
235(7.1)
8(0.2)
91(2.8)
11(0.3)
66(2.0)
175(5.3)1241(45.0)
269(9.8)
146(5.3)
177(6.4)
158(5.7)
3(0.1)
150(5.4)
33(1.2)
291(10.6)
208(7.5)17.3
4.7
2.6
5.0
1.4
0.1
2.6
0.9
8.6
2.2180.678
32.248
27.988
107.075
4.767
1.478
28.341
15.547
198.328
12.771< 0.001*
< 0.001*
< 0.001*
< 0.001*
0.029*
0.224
< 0.001*
< 0.001*
< 0.001*
< 0.001**P < 0.05。 -
[1] 张福杰,赵燕,马烨,等. 中国免费艾滋病抗病毒治疗进展与成就[J]. 中国艾滋病性病,2022,28(1):6-9. [2] E Jennifer,Edelman,Kirsha,et al. The next therapeutic challenge in HIV: Polypharmacy[J]. Drugs Aging,2013,30(8):613-628. doi: 10.1007/s40266-013-0093-9 [3] 中国疾病预防控制中心,中华医学会感染病学分会艾滋病丙型肝炎学组. 中国艾滋病诊疗指南(2021年版)[J]. 中国艾滋病性病,2021,27(11):1182-1201. [4] 凌雪梅,蔡卫平,钟活麟,等. DTG 3TC简化方案真实世界临床疗效与安全性研究[J]. 传染病信息,2022,35(1):51-55. [5] Sophie Abgrall,Suzanne M Ingle,Margaret T May,et al. Durability of first ART regimen and risk factors for modification,interruption or death in HIV-positive patients starting ART in Europe and North America 2002-2009[J]. AIDS,2013,27(5):803-813. doi: 10.1097/QAD.0b013e32835cb997 [6] 韦黎娟. 艾滋病初始抗病毒治疗方案更换原因分析[D]. 南宁: 广西医科大学硕士学位论文, 2019. [7] 陈美玲,吴亚松,赵德才,等. 艾滋病抗病毒治疗的换药率比较及其影响因素[J]. 中华传染病杂志,2017,35(4):193-197. [8] Sun J,Liu L,Shen J,et al. Reasons and risk factors for the initial regimen modification in Chinese treatment-naive patients with HIV infection:A retrospective cohort analysis[J]. PLoS One,2015,10(7):e0133242. doi: 10.1371/journal.pone.0133242 [9] 楼金成,周曾全,劳云飞,等. 含克力芝的ART方案在早期初治成人HIV/AIDS病人中的疗效及终止治疗情况[J]. 中国艾滋病性病,2017,23(9):802-805. [10] Pedro Cahn,Juan Sierra Madero,Jose Ramon Arribas,et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): Week 48 results from two multicentre,double-blind,randomised,non-inferiority,phase 3 trials[J]. Lancet,2019,393(10167):143-155. doi: 10.1016/S0140-6736(18)32462-0 [11] Pedro Cahn,Juan Sierra Madero,Jose Ramon Arribas,et al. Durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment-naive adults with HIV-1 infection:96-week results from the GEMINI-1 and GEMINI-2 randomized clinical trials[J]. J Acquir Immune Defic Syndr,2020,83(3):310-318. doi: 10.1097/QAI.0000000000002275 [12] Woldemedhin B,Wabe N T. The reason for regimen change among HIV/AIDS patients initiated on first Line highly active antiretroviral therapy in Southern Ethiopia[J]. North American Journal of Medical Sciences,2012,4(1):19-23. [13] Scherzer,Estrella,Choi,et al. Association of tenofovir exposure with kidney disease risk in HIV infection[J]. AIDS,2012,26(7):867-875. doi: 10.1097/QAD.0b013e328351f68f [14] Nduka C U,Stranges S,Kimani P K,et al. Is there sufficient evidence for a causal association between antiretroviral therapy and diabetes in HIV-infected patients? A meta-analysis[J]. Diabetes Metab Res Rev,2017,33(6):2902. doi: 10.1002/dmrr.2902 [15] Tseng A,Szadkowski L,Walmsley S,et al. Association of age with polypharmacy and risk of drug interactions with antiretroviral medications in HIV-positive patients[J]. Ann Pharmacother,2013,47(11):1429-1439. doi: 10.1177/1060028013504075 [16] 赵海,潘锋. 艾滋病防治进入全病程管理新时代-访北京协和医院感染内科主任李太生教授[J]. 中国医药科学,2022,12(1):1-5. doi: 10.3969/j.issn.2095-0616.2022.01.001 -





 下载:
下载: