Analysis of Characteristic Indexes of Liver Injury Model Induced by Different Antituberculosis Drugs in Mice
-
摘要:
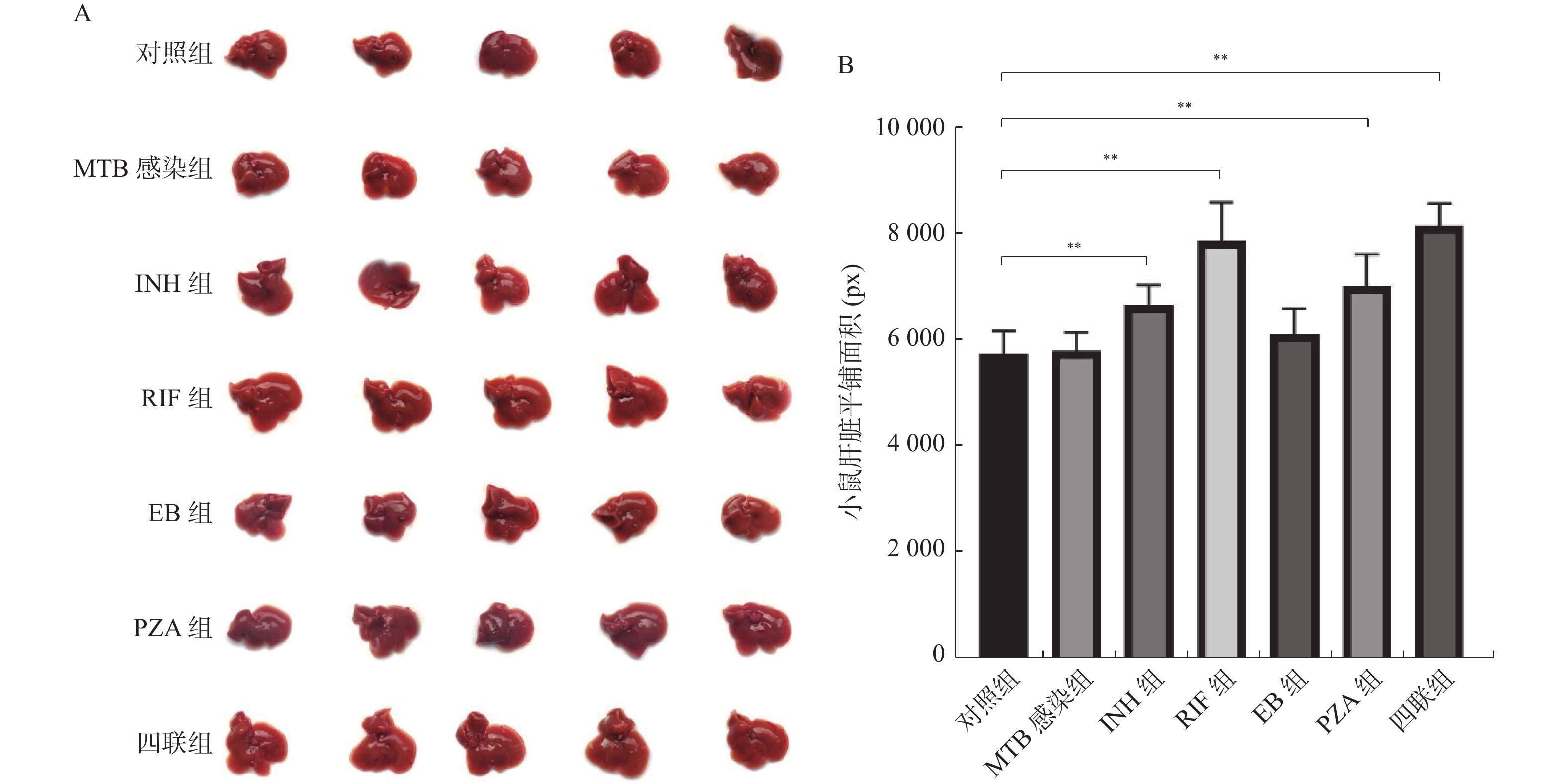
目的 建立抗结核药物性肝损伤小鼠模型,探讨抗结核药物性肝损伤机制,提供动物模型依据。 方法 实验小鼠分为7组,包含对照组,感染结核分枝杆菌(MTB)组,感染MTB后药物灌胃5组:分别灌胃异烟肼(INH)45 mg/(kg·d)、利福平(RIF)90 mg/(kg·d)、乙胺丁醇(EB)135 mg/(kg·d)、吡嗪酰胺(PZA)180 mg/(kg·d)、四联抗结核药物(INH+RIF+EB+PZA),所有给药剂量均按人体剂量与实验动物剂量折算。给药12 h、1~3周后,测定其生化及肝脏指数、病理学指标。 结果 单次INH、RIF、EB、PZA灌胃及四联抗结核药物灌胃12 h后,RIF组及四联组小鼠血清谷丙转氨酶(ALT)及谷草转氨酶(AST)水平升高(P < 0.05)。连续INH、RIF、EB、PZA及四联抗结核药物灌胃1周,INH、RIF、四联组小鼠肝脏血清学指标ALT、AST、TBIL明显升高(P < 0.05)、肝脏指数逐步升高,病变范围增大(P < 0.05)。连续灌药2~3周后,INH、RIF组、四联组小鼠的肝脏血清学指标ALT、AST、GGT、TBIL持续升高(P < 0.05),肝脏指数持续升高(P < 0.05)及病变范围逐步扩大(P < 0.05)。相关性分析显示INH与RIF具有时间累积效应,用药时间与肝损伤血清学指标、肝脏指数、病变范围明显相关(P < 0.05),其余组用药时间与肝损伤指标无明显相关性(P > 0.05)。 结论 四联抗结核药之间有拮抗药物肝脏毒性的趋势,INH及RIF是造成小鼠肝损伤的主导因素,其病理改变以肝细胞损伤为主,且病理指标异常程度早于血清学指标。 Abstract:Objective To establish a mouse model of anti-tuberculous drug-induced liver injury, to explore the mechanism of anti-tuberculous drug induced liver injury, and to provide the basis of animal model. Methods Experimental mice were divided into 7 groups, including control group, group infected with Mycobacterium tuberculosis (MTB), the other 5 groups were given different drugs after infection with MTB, namely isoniazid (INH) 45 mg/(kg·d), rifampicin (RIF) 90 mg/(kg·d), ethambutol (EB)135 mg/(kg·d), pyrazinamide (PZA)180 mg/(kg·d), tetrad antituberculosis drugs (INH+RIF+EB+PZA), respectively. All doses were converted according to human dose and experimental animal dose. After 12 hours and 1-3 weeks of administration, the biochemical, liver index and pathological indexes were measured. Results After a single intragastric administration of INH, RIF, EB, PZA and tetrad anti-tuberculosis drugs for 12 hours, the levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in RIF group and tetrad group mice increased (P < 0.05). After continuous intragastric administration of INH, RIF, EB, PZA and tetrad anti-tuberculosis drugs for 1 week, the serum indexes of ALT, AST and TBIL in the liver of INH, RIF and tetrad group mice were significantly increased (P < 0.05), and the liver index and pathological range were gradually increased and enlarged (P < 0.05). After continuous intragastric administration for 2-3 weeks, the serum indexes of liver ALT, AST, GGT and TBIL in INH, RIF and tetrad groups continued to increase (P < 0.05), the liver index continued to increase (P < 0.05) and the range of lesions expanded (P < 0.05). The correlation analysis showed that INH and RIF had a time cumulative effect, and the time of administration was significantly correlated with the serological index, liver index and lesion range of liver injury (P < 0.05), while the time of administration in other groups was not significantly correlated with the liver injury index (P > 0.05). Conclusion There is a trend of antagonistic liver toxicity among four combination anti-tuberculosis drugs, and INH and RIF are the main factors causing liver damage in mice. The pathological changes are mainly liver cell damage, and the abnormal degree of pathological indicators is earlier than serological indicators. The use of drugs to protect liver cell lesions in advance may have some clinical significance in preventing liver injury caused by anti-tuberculosis drugs. -
Key words:
- Anti-tuberculosis drugs /
- Liver injury /
- Mouse model /
- Indicators /
- Analysis
-
图 2 不同抗结核药物灌胃2周导致的小鼠肝损伤病理变化(40×)
A:对照组小鼠肝组织HE染色图片;B:MTB感染组小鼠肝组织HE染色图片;C:INH组小鼠肝组织HE染色图片;D:RIF组小鼠肝组织HE染色图片;E:EB组小鼠肝组织HE染色图片;F:PZA组小鼠肝组织HE染色图片;G:四联组小鼠肝组织HE染色图片。
Figure 2. Pathological changes of liver injury in mice caused by intragastric administration of different antituberculosis drugs for 2 weeks (40×)
表 1 病理观察结果分级 (%)
Table 1. Classification of pathological observation results (%)
分级 病变分级 病变程度 1 极轻度 < 5 2 轻度 5~25 3 中度 26~50 4 重度 51~75 5 极重度 > 75 表 2 不同抗结核药物灌胃12 h后的小鼠肝脏指标变化(
$\bar x \pm s $ )Table 2. Changes in liver indicators of mice after 12 hours of intragastric administration of different anti-tuberculosis drugs (
$\bar x \pm s $ )类别 剂量(mg/(kg·d)) ALT[M(Q1,Q3)] AST[M(Q1,Q3)] ALP GGT TBIL Live index INH组12 h 45 37.4(31.3,55.5) 41.4(30.5,52.6) 11.7 ± 2.6 39.3 ± 3.8 7.9 ± 2.8 3.4 ± 0.2 RIF组12 h 90 50.3(33.4,72.2)* 66.4(43.2,80.7)* 11.5 ± 2.2 38.9 ± 6.1 8.7 ± 3.1 3.5 ± 0.3 EB组12 h 135 27.7(22.1,37.2) 33.7(24.1,39.7) 10.7 ± 2.5 37.1 ± 4.5 7.4 ± 4.0 3.3 ± 0.2 PZA组12 h 180 33.4(24.1,45.7) 35.3(23.5,43.1) 9.8 ± 3.3 37.8 ± 3.9 7.5 ± 4.0 3.3 ± 0.2 四联组12 h 参照上述剂量 65.0(51.5,81.3)* 78.5(55.1,92.1)* 13.2 ± 2.1 39.6 ± 5.8 8.9 ± 3.8 3.4 ± 0.3 MTB感染组 0 26.4(17.5,32.1) 31.2(23.5,39.2) 9.3 ± 2.3 37.5 ± 4.3 7.6 ± 3.5 3.2 ± 0.3 对照组 0 25.1(17.1,29.7) 29.4(21.3,38.6) 8.9 ± 2.9 36.9 ± 4.7 7.5 ± 3.9 3.2 ± 0.2 H/F 19.655 16.409 0.69 0.576 0.675 2.335 P 0.001* 0.008* 0.781 0.686 0.554 0.152 不同组别中该组与MTB感染组比较,*P < 0.05。 表 3 不同抗结核药物灌胃1周的小鼠肝脏指标变化(
$\bar x \pm s $ )Table 3. Changes in liver indicators of mice after 1 week of intragastric administration of different anti-tuberculosis drugs (
$\bar x \pm s $ )类别 剂量(mg/(kg·d)) ALT[M(Q1,Q3)] AST[M(Q1,Q3)] ALP GGT TBIL Live index 病变范围 INH组1周 5 52.3(36.5,64.3)* 52.2(47.1,66.8)* 18.0 ± 3.3 41.4 ± 5.6 10.7 ± 4.3* 8.3 ± 0.2* 32.3 ± 1.7* RIF组1周 10 67.1(49.6,72.4)* 78.5(59.3,93.1)* 17.9 ± 3.0 42.6 ± 5.6 15.3 ± 5.8* 8.2 ± 0.3* 36.8 ± 1.8* EB组1周 15 31.6(22.8,44.6) 36.2(23.8,49.5) 17.0 ± 3.0 39.1 ± 6.0 8.9 ± 4.8 4.1 ± 0.1 12.2 ± 1.2 PZA组1周 15 37.2(28.1,55.7) 41.3(32.6,47.8) 17.9 ± 3.4 39.7 ± 6.0 8.8 ± 5.5 4.2 ± 0.2 12.0 ± 1.0 四联组1周 参照上述剂量 71.6(57.3,87.1)* 79.5(59.3,88.2)* 19.7 ± 3.0 41.6 ± 7.4 14.8 ± 6.3* 9.0 ± 0.2* 38.6 ± 2.9* MTB感染组 0 25.3(18.3,33.4) 26.2(18.5,33.2) 16.5 ± 3.3 38.6 ± 7.2 8.7 ± 5.5 4.1 ± 0.1 11.4 ± 0.1 对照组 0 24.6(17.2,32.6) 25.1(17.8,32.6) 16.3 ± 3.5 38.3 ± 7.4 8.6 ± 5.7 4.0 ± 0.1 11.2 ± 0.2 H/F 20.405 18.801 1.175 0.057 4.519 3.367 13.247 P 0.001* 0.002* 0.180 0.996 0.007* 0.008* <0.001* 不同组别中该组与MTB感染组比较,*P < 0.05。 表 4 不同抗结核药物灌胃2周的小鼠肝脏指标变化(
$\bar x \pm s $ )Table 4. Changes in liver indicators of mice after 2 weeks of intragastric administration of different anti-tuberculosis drugs (
$\bar x \pm s $ )类别 剂量(mg/(kg·d)) ALT[M(Q1,Q3)] AST[M(Q1,Q3)] ALP GGT TBIL Live index 病变范围 INH组2周 5 61.8(55.2,74.5)* 63.4(42.1,76.5)* 22.4 ± 2.6 42.4 ± 1.3 12.6 ± 3.2* 14.7 ± 0.2* 51.5 ± 1.7* RIF组2周 10 77.2(55.7,91.5)* 86.4(69.9,100.2)* 22.9 ± 2.7 43.1 ± 1.6 17.8 ± 2.3* 15.2 ± 0.6* 57.2 ± 2.3* EB组2周 15 39.8(31.3,46.7) 43.7(33.2,54.1) 19.9 ± 1.9 40.6 ± 1.4 9.2 ± 3.0 5.7 ± 0.5 13.1 ± 0.4 PZA组2周 15 37.3(26.3,42.5) 45.3(37.1,53.7) 19.9 ± 1.6 40.9 ± 2.6 9.1 ± 2.9 5.7 ± 0.4 13.6 ± 0.5 四联组2周 参照上述剂量 64.8(48.7,78.3)* 75.5(57.3,91.4)* 22.8 ± 2.6 42.9 ± 2.4 6.2 ± 2.6 14.8 ± 0.8* 42.9 ± 0.9* MTB感染组 0 24.6(18.6,29.7) 31.3(23.2,38.6) 19.5 ± 1.4 41.2 ± 0.8 8.9 ± 2.3 5.7 ± 0.3 12.8 ± 0.2 对照组 0 22.3(17.5,28.9) 29.4(22.7,37.9) 18.7 ± 1.5 39.9 ± 0.9 8.8 ± 2.4 5.2 ± 0.2 12.6 ± 0.2 H/F 24.203 19.279 1.635 1.037 4.049 9.985 12.291 P <0.001* 0.002* 0.175 0.171 0.004* <0.001* <0.001* 不同组别中该组与MTB感染组比较,*P < 0.05。 表 5 不同抗结核药物灌胃3周的小鼠肝脏指标变化(
$\bar x \pm s $ )Table 5. Changes in liver indicators of mice after 3 weeks of intragastric administration of different anti-tuberculosis drugs (
$\bar x \pm s $ )类别 剂量(mg/(kg·d)) ALT[M(Q1,Q3)] AST[M(Q1,Q3)] ALP GGT TBIL Live index 病变范围 INH组3周 5 77.8(69.7,89.4)* 81.4(71.2,91.4)* 33.4 ± 1.3 59.7 ± 4.3* 26.1 ± 2.5* 17.5 ± 0.7* 56.5 ± 1.9* RIF组3周 10 82.1(67.2,94.1)* 96.4(88.3,104.5)* 33.9 ± 1.7 70.3 ± 2.0* 28.7 ± 4.8* 22.2 ± 0.2* 66.2 ± 2.3* EB组3周 15 42.4(33.4,50.5) 44.3(35.2,59.1) 33.0 ± 3.6 46.9 ± 2.1 22.3 ± 2.3 5.7 ± 0.3 14.6 ± 1.2* PZA组3周 15 39.7(30.2,48.4) 47.3(38.7,54.1) 32.5 ± 3.1 47.1 ± 3.0 22.3 ± 2.8 5.8 ± 0.5 15.0 ± 1.1* 四联组3周 参照上述剂量 66.7(49.2,79.9)* 79.5(65.4,93.5)* 32.9 ± 2.3 53.2 ± 3.9* 26.2 ± 2.5* 15.7 ± 0.4* 45.6 ± 1.6* MTB感染组 0 26.2(20.3,34.3) 38.1(26.4,41.3) 33.4 ± 2.9 44.1 ± 1.8 19.5 ± 0.7 5.5 ± 0.3 5.2 ± 0.3 对照组 0 24.4(19.1,32.6) 36.4(25.2,40.4) 31.1 ± 3.0 43.7 ± 1.9 18.8 ± 0.8 5.3 ± 0.2 4.9 ± 0.2 H/F 23.602 23.913 0.674 12.459 5.156 18.295 24.518 P < 0.001* < 0.001* 0.650 < 0.001* 0.001* < 0.001* < 0.001* 不同组别中该组与MTB感染组比较,*P < 0.05。 表 6 INH组小鼠肝损伤指标与用药时间的相关性分析(
$\bar x \pm s $ )Table 6. Correlation analysis between liver injury indicators and medication time in INH group mice (
$\bar x \pm s $ )类别 ALT[M(Q1,Q3)] AST[M(Q1,Q3)] ALP GGT TBIL Live index 病变范围 INH组12 h 37.4(31.3,55.5) 41.4(30.5,52.6) 11.7 ± 2.6 39.3 ± 3.8 7.9 ± 2.8 3.4 ± 0.2 0 INH组1周 52.3(36.5,64.3) 52.2(47.1,66.8) 18.0 ± 3.3 41.4 ± 5.6 10.7 ± 4.3 8.3 ± 0.2 32.3 ± 1.7 INH组2周 61.8(55.2,74.5) 63.4(42.1,76.5) 22.4 ± 2.6 42.4 ± 1.3 12.6 ± 3.2 14.7 ± 0.2 51.5 ± 1.7 INH组3周 77.8(69.7,89.4) 81.4(71.2,91.4) 33.4 ± 1.3 59.7 ± 4.3 16.1 ± 2.5 17.5 ± 0.7 56.5 ± 1.9 r 0.771 0.637 0.766 0.496 0.435 0.893 0.913 P 0.001* 0.007* < 0.001* 0.005* 0.014* < 0.001* < 0.001* *P < 0.05 。 表 7 RIF组小鼠肝损伤指标与用药时间的相关性分析(
$\bar x \pm s $ )Table 7. Correlation analysis between liver injury indicators and medication time in RIF group mice (
$\bar x \pm s $ )类别 ALT[M(Q1,Q3)] AST[M(Q1,Q3)] ALP GGT TBIL Live index 病变范围 RIF组12 h 50.3(33.4,72.2) 66.4(43.2,80.7) 11.5 ± 2.2 38.9 ± 6.1 8.7 ± 3.1 3.5 ± 0.3 0 RIF组1周 67.1(49.6,72.4) 78.5(59.3,93.1) 17.9 ± 3.0 42.6 ± 5.6 15.3 ± 5.8 8.2 ± 0.3 36.8 ± 1.8 RIF组2周 77.2(55.7,91.5) 86.4(69.9,100.2) 22.9 ± 2.7 43.1 ± 1.6 17.8 ± 2.3 15.2 ± 0.6 57.2 ± 2.3 RIF组3周 82.1(67.2,94.1) 96.4(88.3,104.5) 33.9 ± 1.7 70.3 ± 2.0 28.7 ± 4.8 22.2 ± 0.2 66.2 ± 2.3 r 0.536 0.570 0.870 0.570 0.536 0.905 0.926 P 0.023* 0.016* < 0.001* 0.016* 0.023* < 0.001* < 0.001* *P < 0.05。 -
[1] 李永红,李红恩,雷世鑫,等. 抗结核药致中国人群药物性肝损伤危险因素的Meta分析[J]. 中国抗生素杂志,2021,46(6):628-633. [2] 占旭. 吡嗪酰胺和乙胺丁醇治疗初诊空洞型肺结核的临床疗效及预后[J]. 世界最新医学文摘,2021,21(48):159-160. [3] 向晓雪,艾佳晨,赵科,等. 利福平和异烟肼致小鼠肝损伤模型的特征研究[J]. 中国药理学通报,2019,35(4):586-590. [4] Liu X,Zhao M,Mi J,et al. Protective effect of bicyclol on anti-tuberculosis drug induced liver injury in rats[J]. Molecules,2017,22(4):524. doi: 10.3390/molecules22040524 [5] 张冬,胡宝翠,王小红,等. 抗结核药物致肝损伤动物模型的探讨[J]. 包头医学院学报,2019,35(12):76-79. [6] 中华医学会结核病学分会. 抗结核药物性肝损伤诊治指南(2019年版)[J]. 中华结核和呼吸杂志,2019,42(5):345-356. [7] 杨松,郭建琼,严晓峰. 抗结核药物性肝损伤发生机制的研究进展[J]. 中华结核和呼吸杂志,2019(5):378-381. doi: 10.3760/cma.j.issn.1001-0939.2019.05.012 [8] 赵科,谭婉莹,向菊芳,等. 正交设计探讨一线抗结核药物致小鼠肝损伤相互作用研究[J]. 中药药理与临床,2022,38(2):185-190. [9] 邵爽,刘春燕,高沿航. 水飞蓟宾治疗药物性肝损伤的研究进展[J]. 临床肝胆病杂志,2017,33(6):1179-1182. [10] Sharma V,Kaur R,Sharma V L. Ameliorative potential of Adhatoda vasica against anti-tubercular drugs induced hepatic impairments in female Wistar rats in relation to oxidative stress and xeno-metabolism[J]. Journal of Ethnopharmacology: An Interdisciplinary Journal Devoted to Bioscientific Research on Indigenous Drugs,2021,270(1):110-113. [11] 王蓉,韩亮,赵戈蕾. 夏枯草硫酸多糖对异烟肼所致小鼠抗结核药物性肝损伤的保护作用及机制[J]. 临床消化病杂志,2021,33(4):242-245. [12] 贾云鹏,徐杰,马玉,等. EZH2对抗结核药物性肝损伤小鼠Nrf2-ARE通路的影响[J]. 安徽医科大学学报,2021,56(2):233-238. [13] 何玲,唐简,彭忠田. 基于HMGB1-RAGE信号通路研究奥拉米特预防抗结核药物性肝损伤的作用机制[J]. 中国药房,2021,32(18):2229-2235. [14] 刘海涛,张雷鸣,李经纬. 药物性肝损伤患者临床与病理学特征分析[J]. 实用肝脏病杂志,2022,25(4):508-511. doi: 10.3969/j.issn.1672-5069.2022.04.014 -






 下载:
下载: