Effects of Euonymine on Proliferation,Migration,and Cell Cycle of HUVECs and VSMCs
-
摘要:
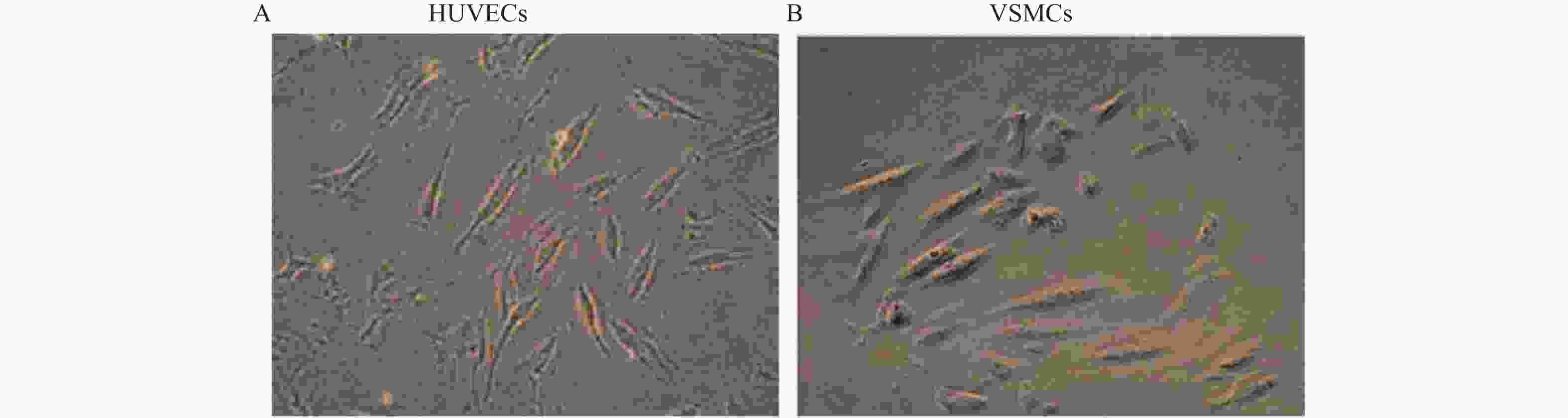
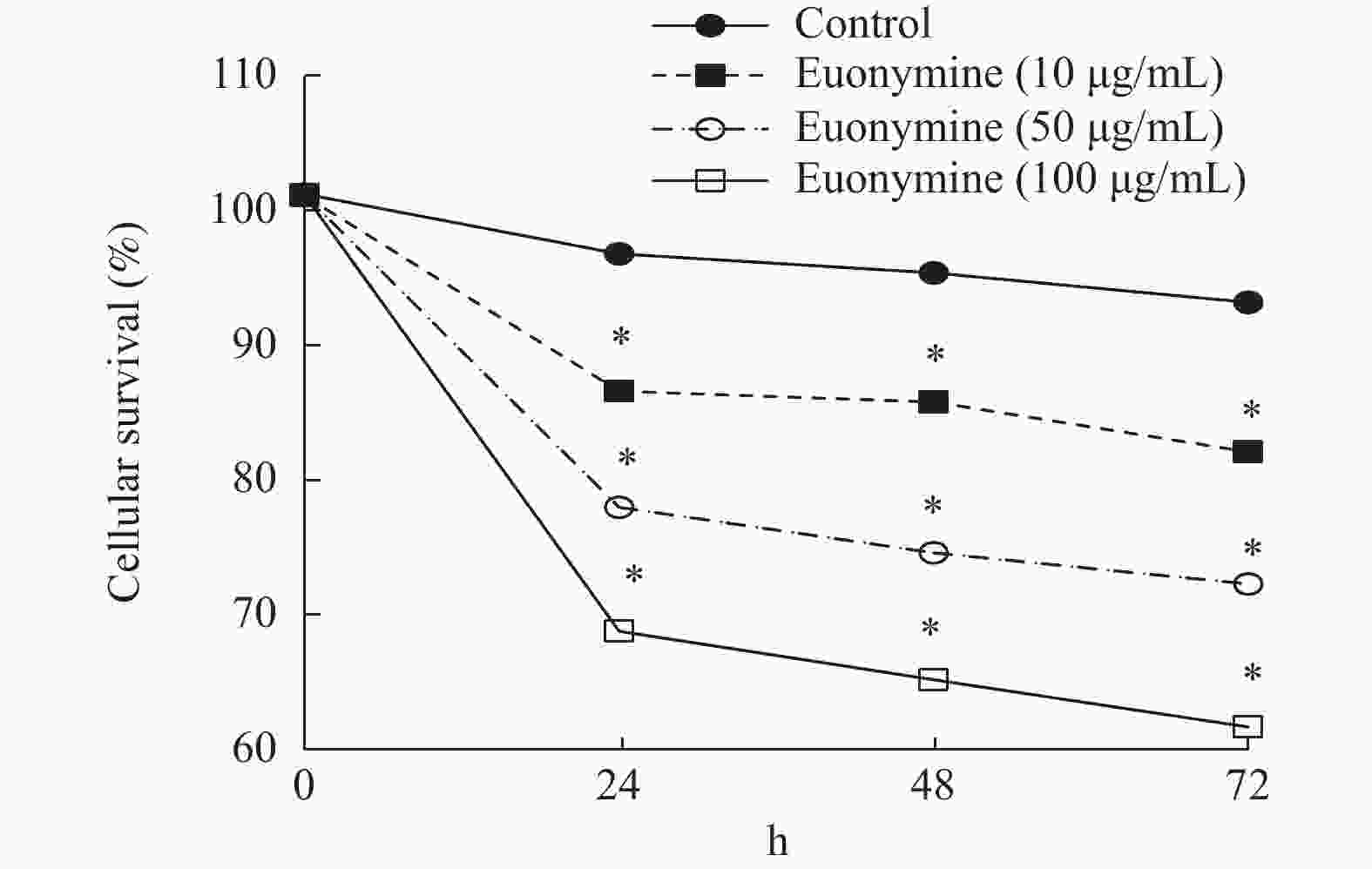
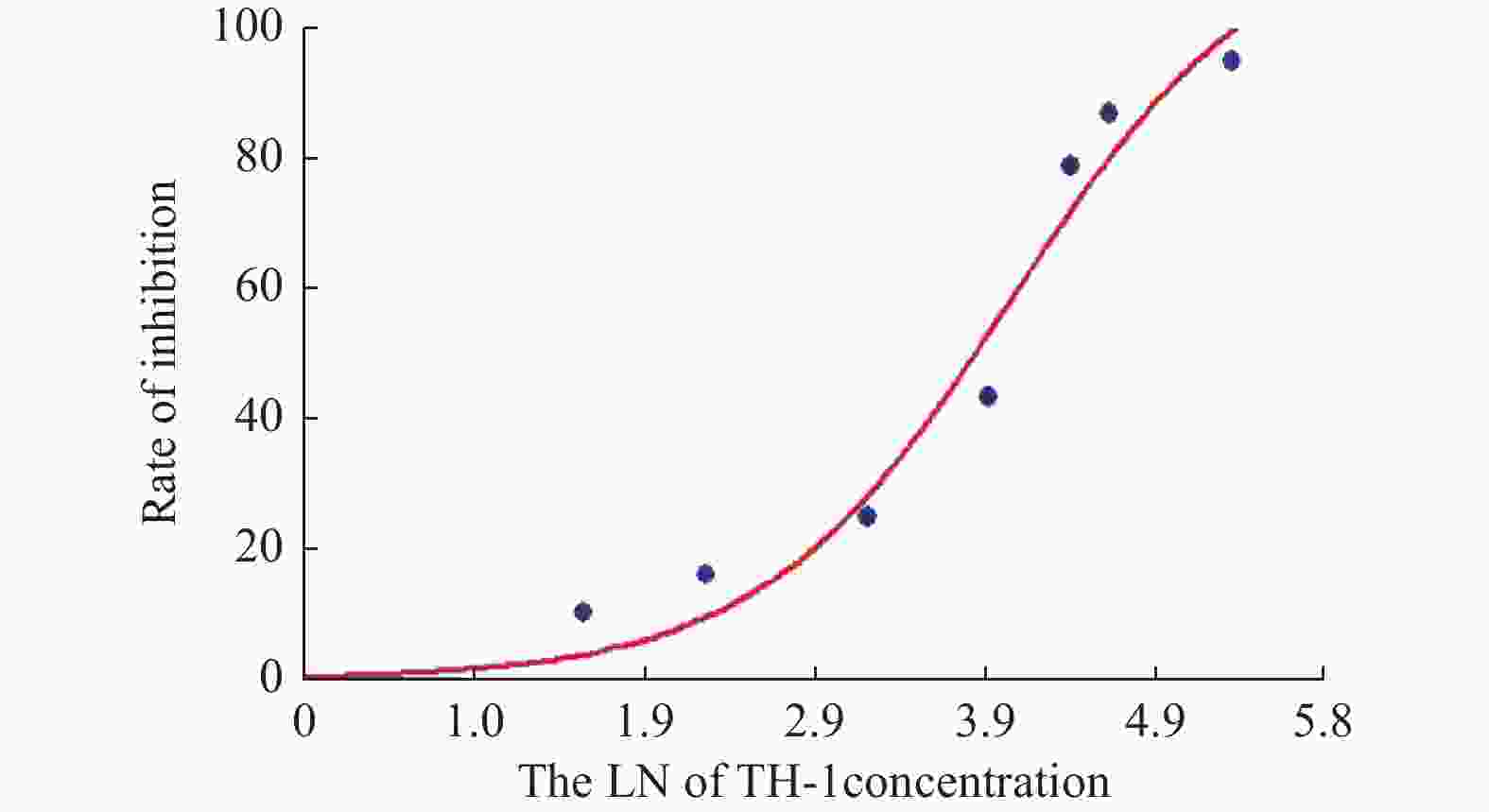
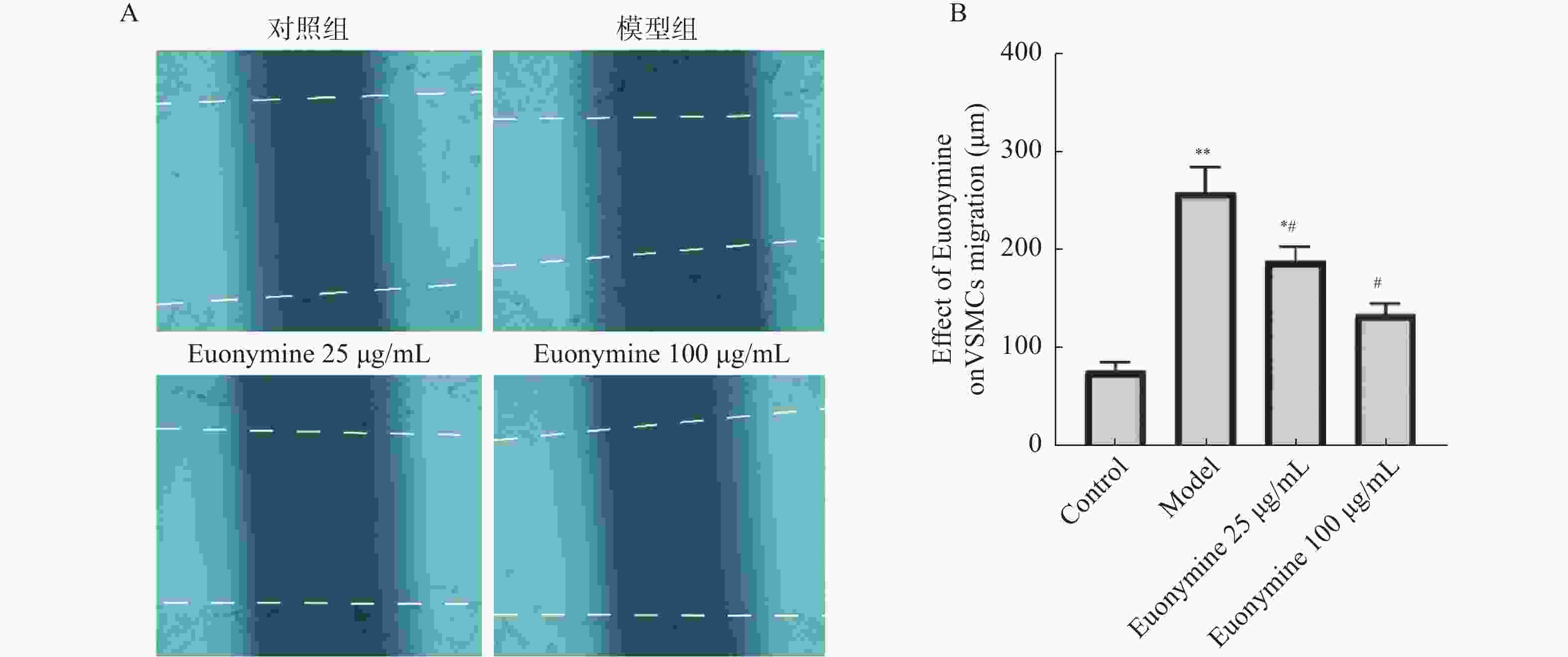
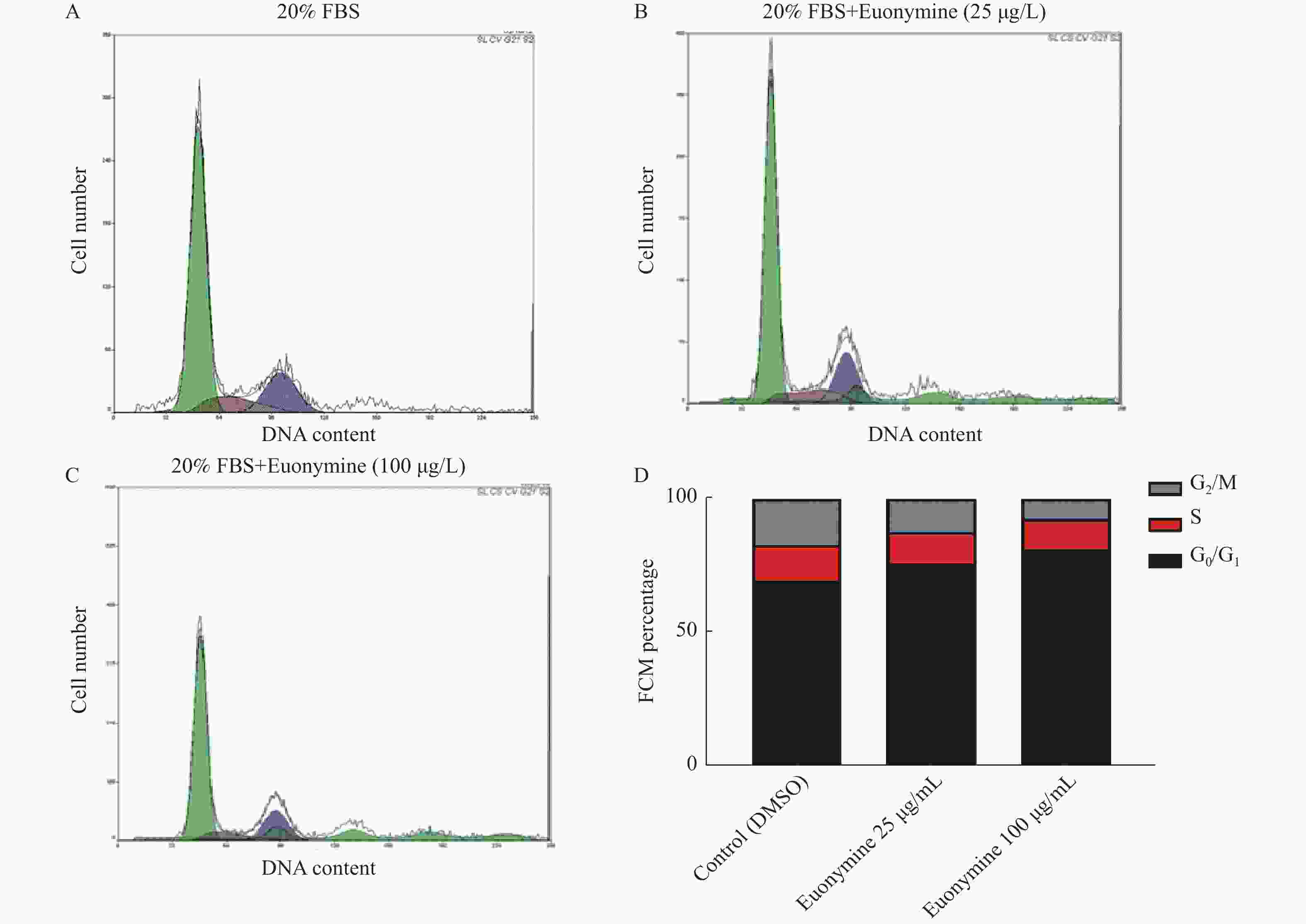
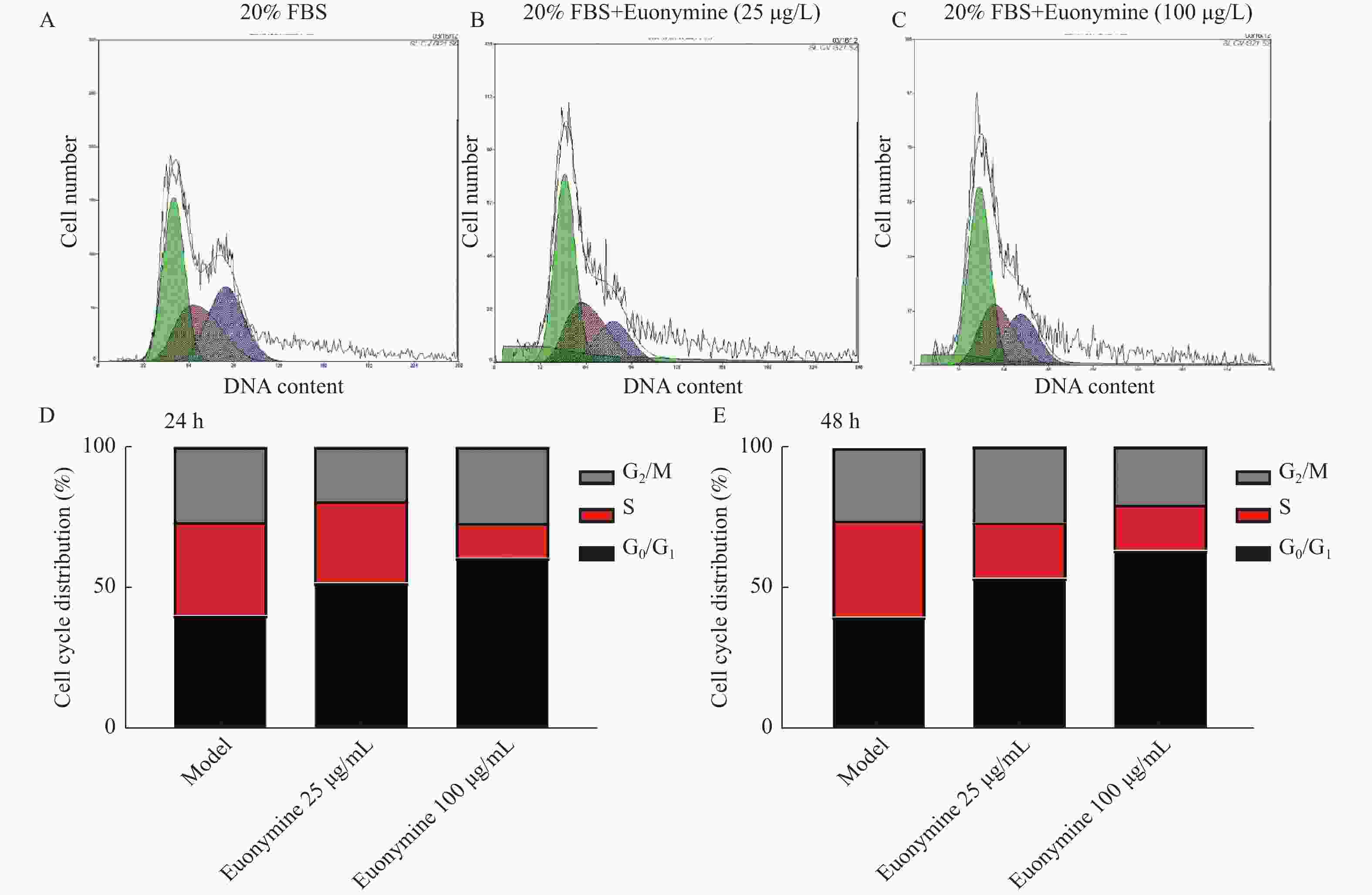
目的 探讨卫茅碱(Euonymine)对人脐静脉内皮细胞(HUVECs)及人血管平滑肌细胞(VSMCs)的影响,以明确其防治冠脉支架内再狭窄(in-stent restenosis,ISR)的可能机制。 方法 台盼蓝检测Euonymine对HUVECs成活率的影响;MTT法检测Euonymine对HUVECs及VSMCs增殖的影响;划痕法检测Euonymine对VSMCs迁移的影响。 结果 MTT结果显示,卫茅碱干预后, HUVECs和VSMCs的吸光度降低。另外,Euonymine分别作用HUVECs 24 h,VSMCs 48 h后,测的半数有效抑制浓度(IC50)分别为28.6 µg/mL和33.92 µg/mL。流式细胞结果显示,Euonymine(25 μg/mL)使HUVECs和VSMCs的细胞周期阻滞于G0/G1期,而Euonymine(100 µg/mL)使VSMCs有丝分裂被阻断于G0/G1期及G2/M期。 结论 Euonymine主要通过抑制VSMCs的增殖和迁移,影响细胞周期,对于抑制经皮冠状动脉介入治疗术(percutaneous coronary intervention,PCI)后新生内膜形成有潜在的临床应用价值。 Abstract:Objective To investigate the effects of Euonymine on isolated cultured human umbilical vein endothelial cells (HUVECs) and human vascular smooth muscle cells (VSMCs), so as to elucidate the possible mechanism of Euonymine in prevention and treatment of ISR. Methods The effect of trypan blue assay on the viability of HUVECs. MTT assay was used to observe the effect of Euonymine on the proliferation of normal HUVECs and VSMCs. A wound healing assay was performed to detect the effect of Euonymine on the migration of VSMCs. Results The MTT results showed that the absorbance of HUVECs and VSMCs decreased after the Euonymine intervention. In addition, the IC50 of Euonymine on HUVECs after 24 hours and on VSMCs after 48 hours were 28.6 µg/mL and 33.92 µg/mL, respectively. Flow cytometry results showed that Euonymine (25 μg/mL) arrested the cell cycle of both HUVECs and VSMCs at the G0/G1 phase, while Euonymine (100 μg/mL) blocked the mitosis of VSMCs at both the G0/G1 and G2/M phases. Conclusion Euonymine affects the cell cycle mainly by inhibiting the proliferation and migration of VSMCs and has potential value of clinical applications in inhibiting neointima hyperplasia after percutaneous coronary intervention (PCI). -
Key words:
- Euonymine /
- HUVECs /
- VSMCs /
- Anti-proliferative /
- Anti-migratory /
- Cell cycle
-
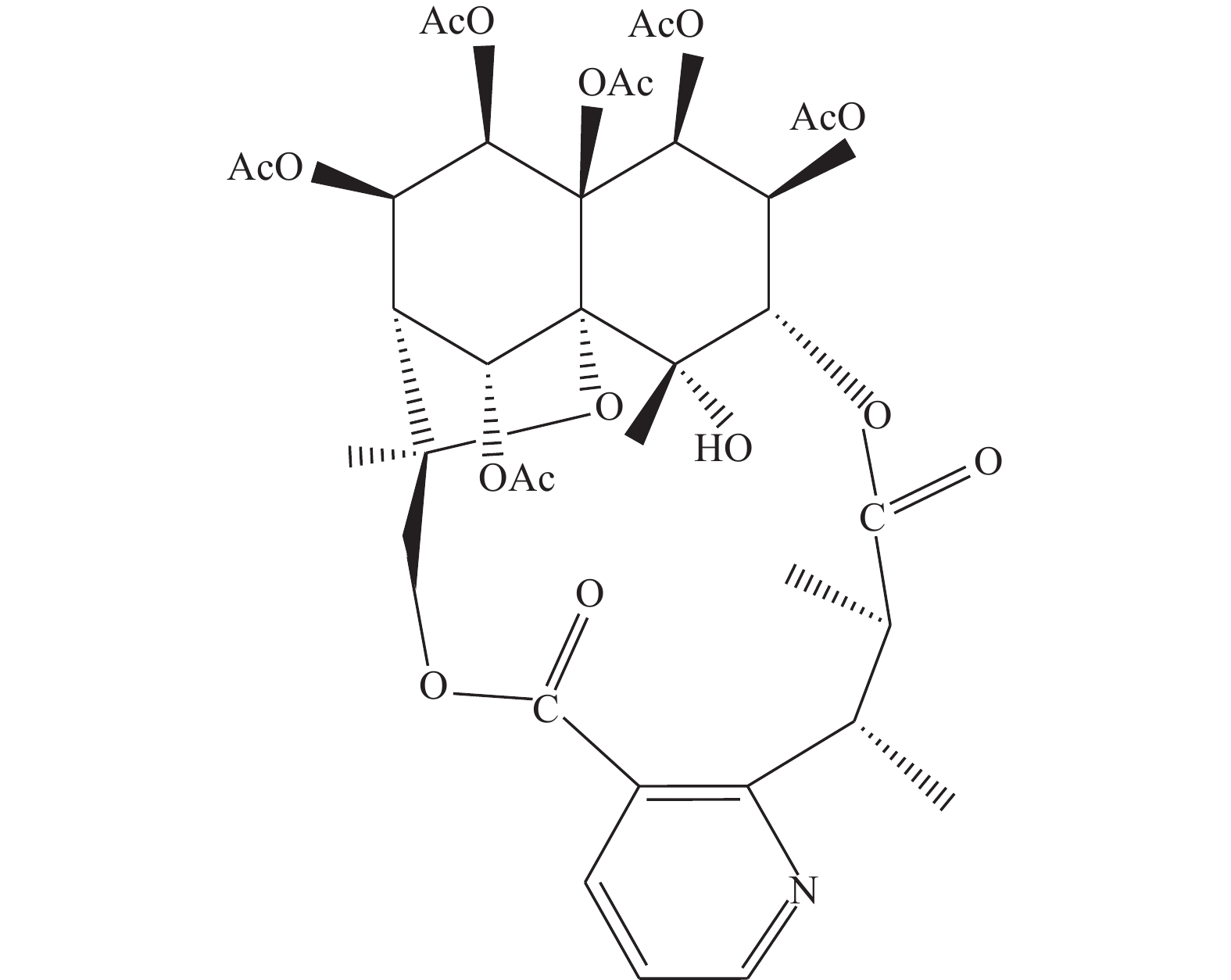
图 1 卫茅碱的化学结构式[13]
Figure 1. Chemical structural of Euonymine
表 1 不同浓度Euonymine对HUVECs增殖的影响(
$ \bar x \pm s $ ,n = 6)Table 1. Effect of different concentrations of Euonymine on the proliferation of HUVECs (
$\bar x \pm s $ ,n = 6)分组 OD (570 nm) IR(%) F P 正常对照组 DMSO 0.768 ± 0.06 Euonymine(µg/mL) 12.5 0.654 ± 0.08△ 16.1 25 0.591 ± 0.19△ 25.0 5.97 0.030* 50 0.460 ± 0.07△ 43.5 100 0.152 ± 0.12△ 87.0 *P < 0.05;与正常对照组相比较,△P < 0.05。 表 2 不同浓度Euonymine对VSMCs增殖的影响(
$\bar x \pm s$ ,n = 6)Table 2. Effect of different concentrations of Euonymine on the proliferation of VSMCs (
$\bar x \pm s $ ,n = 6)分组 OD (570 nm) 24 h 48 h 72 h 96 h 正常对照组 DMSO 0.788 ± 0.16 0.841 ± 0.14 0.885 ± 0.08 0.925 ± 0.20 Euonymine
(µg/mL)12.5 0.688 ± 0.10 0.680 ± 0.13 0.676 ± 0.12△ 0.637 ± 0.04 25 0.626 ± 0.08△ 0.628 ± 0.19△ 0.607 ± 0.10△ 0.569 ± 0.12△ 50 0.524 ± 0.11△ 0.425 ± 0.15△ 0.419 ± 0.07△ 0.402 ± 0.18△ 100 0.312 ± 0.07△ 0.259 ± 0.13△ 0.219 ± 0.17△ 0.188 ± 0.08△ 200 0.259 ± 0.04△ 0.185 ± 0.16△ 0.156 ± 0.06△ 0.137 ± 0.18△ F 6.21 6.49 6.61 6.76 P 0.041* 0.041* 0 .031* 0.028* *P < 0.05;与正常对照组比较,△P < 0.05。 -
[1] Jensen L O,Thayssen P,Thuesen L,et al. Influence of a pressure gradient distal to implanted bare-metal stent on in—stent restenosis after percutaneous coronary intervention[J]. Circulation,2007,116(24):2802-2808. doi: 10.1161/CIRCULATIONAHA.107.704064 [2] Maarten J S,Gert J L,Braim M R,et al. Primary Tenting of Totally Occluded Native Coronary Arteries II (PRISON II): A randomized comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusicms[J]. Circulation,2006,114(9):921-928. doi: 10.1161/CIRCULATIONAHA.106.613588 [3] Kawai K,Virmani R,Finn A V,et al. In-Stent Restenosis[J]. Interv Cardiol Clin,2022,11(4):429-443. [4] Stettler C,Wandel S,Allemann S,et al. Outcomes associated with drug-eluting and bare-metal stents: A collaborative network meta-analysis[J]. Lancet,2007,370(9591):937-948. doi: 10.1016/S0140-6736(07)61444-5 [5] Hong S J,Hong M K. Drug-eluting stents for the treatment of coronary artery disease: A review of recent advances[J]. Expert Opinion on Drug Delivery,2022,19(3):269-280. doi: 10.1080/17425247.2022.2044784 [6] Hu W and Jiang J. Hypersensitivity and in-stent restenosis in coronary stent materials[J]. Front Bioeng Biotechnol,2022,10:1003322. doi: 10.3389/fbioe.2022.1003322 [7] Hsiao S T,Spencer T,Boldock L,et al. Endothelial repair in stented arteries is accelerated by inhibition of Rho-associated protein kinase[J]. Cardiovasc Res,2016,112(3):689-701. doi: 10.1093/cvr/cvw210 [8] Guo L W,Wang B,Goel S A,et al. Halofuginone stimulates adaptive remodeling and preserves re-endothelialization in balloon-injured rat carotid arteries[J]. Circ Cardiovasc Interv,2014,7(4):594-601. doi: 10.1161/CIRCINTERVENTIONS.113.001181 [9] Alraies M C,Darmoch F,Tummala R,et al. Diagnosis and management challenges of in-stent restenosis in coronary arteries[J]. World J Cardiol,2017,9(8):640-651. doi: 10.4330/wjc.v9.i8.640 [10] Yang F,Chen Q,He S,et al. MiR-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation[J]. Circulation,2018,137(17):1824-1841. doi: 10.1161/CIRCULATIONAHA.117.027799 [11] Glogov S. Intimal hyplasia,vascular remodeling and the restenosis problem[J]. Circulation,1993,88:152-158. [12] Zhao J,Zhang F,Xiao X,et al. Tripterygium hypoglaucum (Levl. ) hutch and its main bioactive components: Recent advances in pharmacological activity,pharmacokinetics and potential toxicity[J]. Front Pharmacol,2021,12:715359. doi: 10.3389/fphar.2021.715359 [13] Zhang L,Tao Y,Yang R,et al. Euonymine inhibits in-stent restenosis through enhancing contractile phenotype of vascular smooth muscle cells via modulating the PTEN/AKT/mTOR signaling pathway[J]. Phytomedicine,2022,107:154450. doi: 10.1016/j.phymed.2022.154450 [14] 陈妍. 昆明山海棠提取物对人血管内皮及血管平滑肌细胞增殖的影响[D]. 昆明: 昆明医科大学, 2012. [15] Pasterkamp G,Ruijter H M,Libby P. Temporal shifts in clinical presentation and underlying mechanisms of atherosclerotic disease[J]. Nat Rev Cardiol,2017,14(1):21-29. doi: 10.1038/nrcardio.2016.166 [16] Heiden K V,Gijsen F J,Narracott A,et al. The effects of stenting on shear stress: Relevance to endothelial injury and repair[J]. Cardiovasc Res,2013,99(2):269-275. doi: 10.1093/cvr/cvt090 [17] Kanellakis P,Nestel P,Bobik A. Angioplasty-induced superoxide anions and neointimal hyperplasia in the rabbit carotid artery:Suppression by the isoflavone trans-tetrahydrodaidzei[J]. Atherosclerosis,2004,176(1):63-72. [18] Lim W W,Corden B,Ng B,et al. Interleukin-11 is important for vascular smooth muscle phenotypic switching and aortic inflammation,fibrosis and remodeling in mouse models[J]. Sci Rep,2020,10(1):17853. doi: 10.1038/s41598-020-74944-7 [19] Karacsonyi J,Sasi V,Ung I,et al. Management of a balloon shaft fracture during subintimal retrograde chronic total occlusion percutaneous coronary intervention due to in-stent restenosis[J]. J Invasive Cardiol,2018,30(8):E64-E66. [20] Nicolais C,Lakhter V,Virk H,et al. Therapeutic Options for In-Stent Restenosis[J]. Curr Cardiol Rep,2018,20(2):7-21. doi: 10.1007/s11886-018-0952-4 [21] Benada J,Macurek L. Targeting the checkpoint to kill cancer cells[J]. Biomolecules,2015,5(3):1912-1937. doi: 10.3390/biom5031912 [22] 沈晓君,魏群,陈芳,等. 葛根素对血管平滑肌细胞周期相关蛋白表达的影响[J]. 中国中医基础医学杂志,2011,7(1):69-79. doi: 10.19945/j.cnki.issn.1006-3250.2011.01.032 [23] Altesha M A,Ni T,Khan A,et al. Circular RNA in cardiovascular disease[J]. J Cell Physiol,2019,234(5):5588-5600. doi: 10.1002/jcp.27384 [24] Jamasbi E,Hamelian M,Hossain M A,et al. The cell cycle,cancer development and therapy[J]. Mol Biol Rep,2022,49(11):10875-10883. doi: 10.1007/s11033-022-07788-1 [25] Men H,Cai H,Cheng Q,et al. The regulatory roles of p53 in cardiovascular health and disease[J]. Cell Mol Life Sci,2021,78(5):2001-2018. [26] Rieger A M. Flow cytometry and cell cycle analysis: An Overview[J]. Methods Mol Biol,2022,2579:47-57. [27] Song P,Zhao Q,Zou M H. Targeting senescent cells to attenuate cardiovascular disease progression[J]. Ageing Res Rev,2020,60:101072. doi: 10.1016/j.arr.2020.101072 [28] Wang Z. Cell cycle progression and synchronization: An overview[J]. Methods Mol Biol,2022,2579:3-23. [29] Minzer S,Losno R A,Casas R. The effect of alcohol on cardiovascular risk factors: Is there new information?[J]. Nutrients,2020,12(4):912-918. -






 下载:
下载: