Effect of miR-29c-3p/IGF1 Molecular Axis on Activation,Proliferation and Apoptosis of Hepatic Stellate Cells
-
摘要:
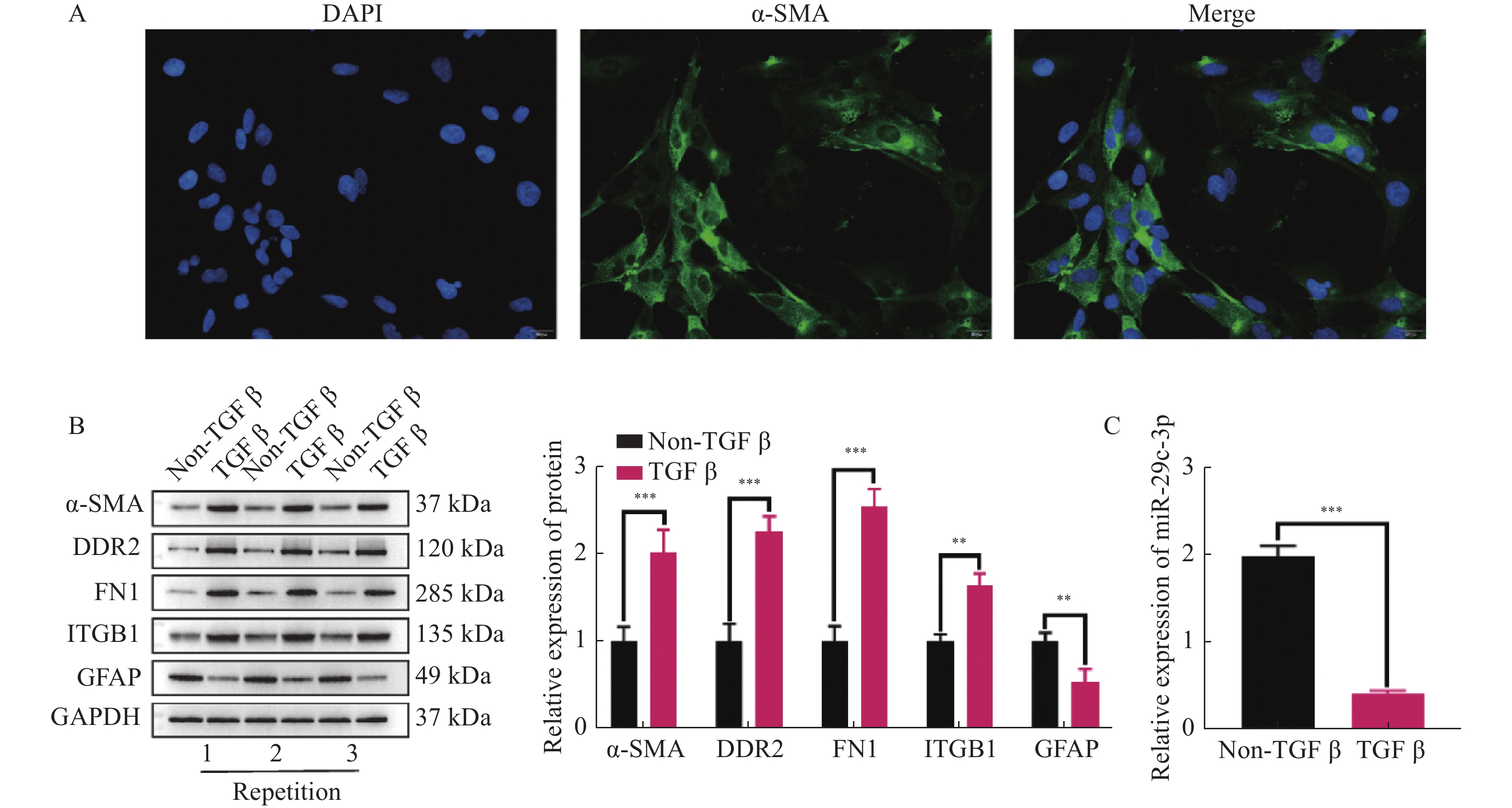
目的 探讨miR-29c-3p通过IGF-1对肝星状细胞(hepatic stellate cells,HSCs)活化,增殖和凋亡的影响。 方法 原代培养小鼠HSCs,并通过免疫荧光检测HSCs标志物ɑ-SMA表达。双萤光素酶报告基因实验验证miR-29c-3p和IGF-1的靶向关系。TGF-β激活HSCs,并且外源性调控miR-29c-3p和IGF-1的表达水平后,分别采用Westernbolt,CCK-8,克隆形成实验和流式细胞术检测活化HSCs中活化相关蛋白(ɑ-SMA,DDR2,FN1,ITGB1和GFAP)的表达,增殖,克隆形成数和凋亡。 结果 ɑ-SMA阳性表达表明成功分离小鼠HSCs。miR-29c-3p mimic可降低野生型IGF-1的萤光素酶活性,但是对突变型IGF-1没有影响。过表达miR-29c-3p和低表达IGF-1能减少ɑ-SMA,DDR2,FN1和ITGB1表达,增加GFAP的表达,并且降低HSCs的增殖活力和克隆形成数,上调其凋亡比例。 结论 miR-29c-3p通过靶向抑制IGF-1表达,进而抑制HSCs活化和增殖,并促进其凋亡。 Abstract:Objective To investigate the effects of miR-29c-3p on the activation, proliferation and apoptosis of hepatic stellate cells (HSCs) via IGF-1. Methods Primary mouse HSCs were cultured and the expression of HSCs marker ɑ-SMA was detected by immunofluorescence. By dual-luciferase reporter gene assay was conducted to Validate the Targeting Relationship between miR-29c-3p and IGF-1. After TGF-β activation of HSCs and exogenous regulation of miR-29c-3p and IGF-1 expression, the expression of activation-related proteins (ɑ-SMA, DDR2, FN1, ITGB1 and GFAP), proliferation, colony-forming number and apoptosis in activated HSCs were detected by Western bolt, CCK-8, colony-forming unit assays and flow cytometry, respectively. Results Positive expression of ɑ-SMA indicated that the successful isolation of mouse HSCs. miR-29c-3p significantly reduced the luciferase activity of wild-type IGF-1, but had no effect on mutant IGF-1. Over-expression of miR-29c-3p and hypo-expression of IGF-1 significantly decreased ɑ-SMA, DDR2, FN1 and ITGB1 expression, increased GFAP expression, and decreased proliferation viability and colony-forming number of HSCs and upregulated their apoptotic ratio. Conclusion miR-29c-3p inhibits the activation and proliferation of HSCs and promotes their apoptosis by targeting IGF-1 expression. -
Key words:
- Alcoholic liver disease /
- Hepatic stellate cells /
- Activation /
- Proliferation /
- Apoptosis /
- miR-29c-3p /
- IGF-1
-
图 2 miR-29c-3p抑制HSCs的活化和增殖,并促进其凋亡
A:采用RT-qPCR检测miR-29c-3p mimic的转染效率;B:通过WB检测miR-29c-3p对活化相关蛋白(ɑ-SMA,DDR2,FN1,ITGB1和GFAP)表达的影响;C:CCK-8试剂盒检测不同组别中TGF-β激活的HSCs增殖活力;D:克隆形成实验检测miR-29c-3p对活化HSCs的克隆形成数的影响;E:流式细胞术检测活化HSCs的凋亡比例。**P < 0.01,***P < 0.001。
Figure 2. miR-29c-3p inhibits the activation and proliferation of HSCs and promotes their apoptosis.
图 4 miR-29c-3p通过IGF-1抑制HSCs的活化和增殖,并促进其凋亡
A: miR-29c-3p inhibior的转染效率;B:通过WB检测得到的sh-IGF-1的转染效率;C:不同组别HSCs中活化相关蛋白(ɑ-SMA,DDR2,FN1,ITGB1和GFAP)表达的表达变化;D:CCK-8实验检测活化的HSCs增殖活力;E:克隆形成实验得到的不同组别中活化HSCs的克隆形成数;F:流式细胞术检测活化HSCs的凋亡比例。与sh-NC组比较,aP < 0.05,aaP < 0.01,aaaP < 0.001;与sh-IGF-1组比较,bP < 0.05,bbP < 0.01,bbbP < 0.001;*P < 0.05,***P < 0.001。
Figure 4. miR-29c-3p inhibits the activation and proliferation of HSCs and promotes their apoptosis through IGF-1.
-
[1] Seitz H K,Bataller R,Cortez-Pinto H,et al. Alcoholic liver disease[J]. Nat Rev Dis Primers,2018,4(1):16. doi: 10.1038/s41572-018-0014-7 [2] 曾赏,李三强,李前辉. 酒精性肝病的研究进展[J]. 世界华人消化杂志,2022,30(12):535-540. [3] 阿比丹·拜合提亚尔,郭津生. 肝纤维化发生时活化肝星状细胞的代谢改变[J]. 中国细胞生物学学报,2021,43(10):2054-2060. [4] Teschke R. Alcoholic liver disease: Current mechanistic aspects with focus on their clinical relevance[J]. Biomedicines,2019,7(3):68. doi: 10.3390/biomedicines7030068 [5] Kordes C,Bock H H,Reichert D,et al. Hepatic stellate cells: Current state and open questions[J]. Biol Chem,2021,402(9):1021-1032. doi: 10.1515/hsz-2021-0180 [6] Bataller R,Brenner D A. Liver fibrosis[J]. J Clin Invest,2005,115(2):209-218. doi: 10.1172/JCI24282 [7] Bartel D P. MicroRNAs: Target recognition and regulatory functions[J]. Cell,2009,136(2):215-233. doi: 10.1016/j.cell.2009.01.002 [8] Michlewski G,Cáceres J F. Post-transcriptional control of miRNA biogenesis[J]. Rna,2019,25(1):1-16. doi: 10.1261/rna.068692.118 [9] Szabo G,Bala S. MicroRNAs in liver disease[J]. Nat Rev Gastroenterol Hepatol,2013,10(9):542-552. doi: 10.1038/nrgastro.2013.87 [10] 安召宏,钟庆,徐启云,等. 肝星状细胞活化和肝细胞性肝癌发生发展中的表观遗传学研究进展[J]. 中国组织化学与细胞化学杂志,2020,29(3):282-286. [11] Pant K,Venugopal S K. Circulating microRNAs: Possible role as non-invasive diagnostic biomarkers in liver disease[J]. Clin Res Hepatol Gastroenterol,2017,41(4):370-377. doi: 10.1016/j.clinre.2016.11.001 [12] Zhang Y J,Hu Y,Li J,et al. Roles of microRNAs in immunopathogenesis of non-alcoholic fatty liver disease revealed by integrated analysis of microRNA and mRNA expression profiles[J]. Hepatobiliary Pancreat Dis Int,2017,16(1):65-79. doi: 10.1016/S1499-3872(16)60098-X [13] Hosseini N,Shor J,Szabo G. Alcoholic hepatitis: A review[J]. Alcohol Alcohol,2019,54(4):408-416. doi: 10.1093/alcalc/agz036 [14] Khomich O,Ivanov A V,Bartosch B. Metabolic hallmarks of hepatic stellate cells in liver fibrosis[J]. Cells,2019,9(1):24. doi: 10.3390/cells9010024 [15] Teschke R. Alcoholic liver disease: Alcohol metabolism,cascade of molecular mechanisms,cellular targets,and clinical aspects[J]. Biomedicines,2018,6(4):106. doi: 10.3390/biomedicines6040106 [16] Kisseleva T,Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression[J]. Nat Rev Gastroenterol Hepatol,2021,18(3):151-166. doi: 10.1038/s41575-020-00372-7 [17] Shan L,Jiang T,Ci L,et al. Purine signaling regulating HSCs inflammatory cytokines secretion,activation,and proliferation plays a critical role in alcoholic liver disease[J]. Mol Cell Biochem,2020,466(1-2):91-102. doi: 10.1007/s11010-020-03691-0 [18] Chen W,Yan X,Yang A,et al. miRNA-150-5p promotes hepatic stellate cell proliferation and sensitizes hepatocyte apoptosis during liver fibrosis[J]. Epigenomics,2020,12(1):53-67. doi: 10.2217/epi-2019-0104 [19] Chen N,Luo J,Hou Y,et al. miR-29c-3p promotes alcohol dehydrogenase gene cluster expression by activating an ADH6 enhancer[J]. Biochem Pharmacol,2022,203(4):115182. [20] Kilikevicius A,Meister G,Corey D R. Reexamining assumptions about miRNA-guided gene silencing[J]. Nucleic Acids Res,2022,50(2):617-634. doi: 10.1093/nar/gkab1256 [21] Wang X,He Y,Mackowiak B,et al. MicroRNAs as regulators,biomarkers and therapeutic targets in liver diseases[J]. Gut,2021,70(4):784-795. doi: 10.1136/gutjnl-2020-322526 [22] Dichtel L E,Cordoba-Chacon J,Kineman R D. Growth hormone and insulin-like growth factor 1 regulation of nonalcoholic fatty liver disease[J]. J Clin Endocrinol Metab,2022,107(7):1812-1824. doi: 10.1210/clinem/dgac088 [23] Cristin L,Montini A,Martinino A,et al. The role of growth hormone and insulin growth factor 1 in the development of non-alcoholic steato-hepatitis: A systematic review[J]. Cells,2023,12(4):517. doi: 10.3390/cells12040517 [24] Adamek A,Kasprzak A. Insulin-like growth factor (IGF) system in liver diseases[J]. Int J Mol Sci,2018,19(5):1308. doi: 10.3390/ijms19051308 [25] Stanley T L,Fourman L T,Zheng I,et al. Relationship of IGF-1 and IGF-binding proteins to disease severity and glycemia in nonalcoholic fatty liver disease[J]. J Clin Endocrinol Metab,2021,106(2):e520-e533. [26] Takahashi Y. The role of growth hormone and insulin-like growth factor-I in the liver[J]. Int J Mol Sci,2017,18(7):1447. doi: 10.3390/ijms18071447 [27] De La Garza R G,Morales-Garza L A,Martin-Estal I,et al. Insulin-like growth factor-1 deficiency and cirrhosis establishment[J]. J Clin Med Res,2017,9(4):233-247. doi: 10.14740/jocmr2761w [28] Martín-González C,González-Reimers E,Quintero-Platt G,et al. Soluble α-klotho in liver cirrhosis and alcoholism[J]. Alcohol Alcohol,2019,54(3):204-208. doi: 10.1093/alcalc/agz019 [29] Luo P,Zheng M,Zhang R,et al. S-Allylmercaptocysteine improves alcoholic liver disease partly through a direct modulation of insulin receptor signaling[J]. Acta Pharm Sin B,2021,11(3):668-679. doi: 10.1016/j.apsb.2020.11.006 [30] Møller S,Becker U,Juul A,et al. Prognostic value of insulinlike growth factor I and its binding protein in patients with alcohol-induced liver disease. EMALD group[J]. Hepatology,1996,23(5):1073-1078. doi: 10.1002/hep.510230521 -






 下载:
下载: