Effects of Periplaneta americana Extract on Autophagy and Invasive Metastasis of Subcutaneous Transplanted Hepatocellular Carcinoma in Nude Mice
-
摘要:
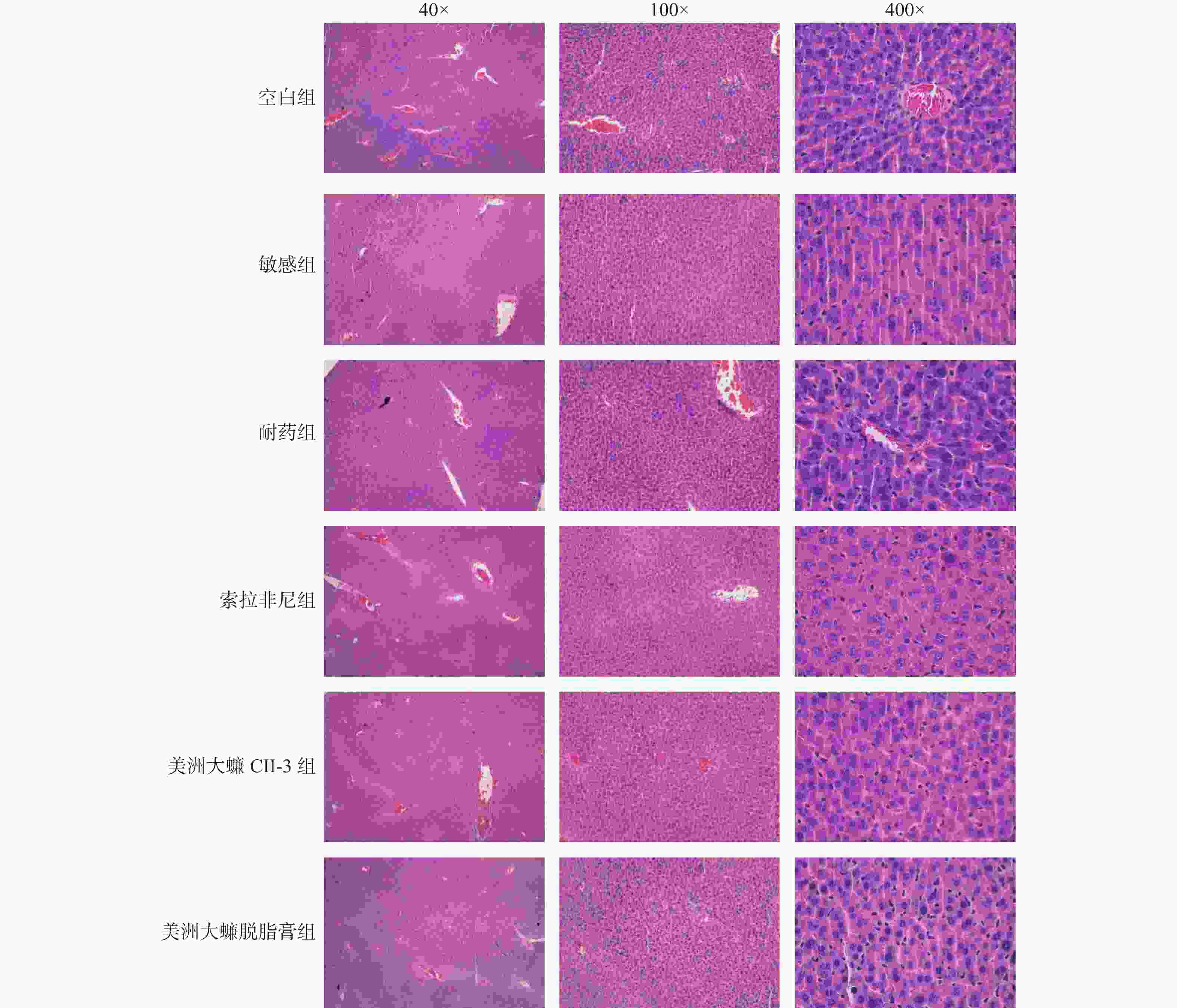
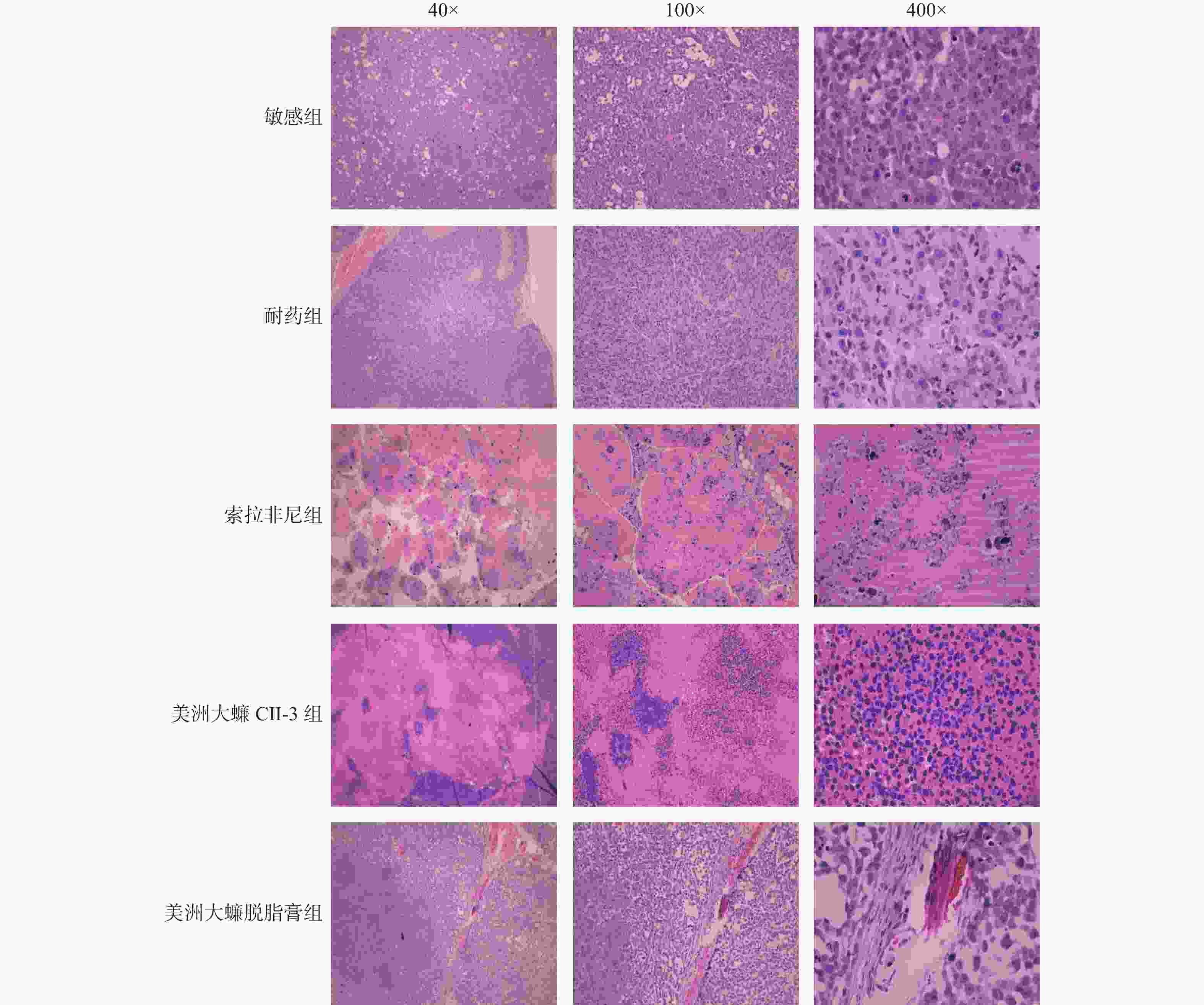
目的 研究美洲大蠊提取物CII-3和脱脂膏对肝癌耐药细胞BEL-7402/5-FU自噬和侵袭转移的影响以及初步的作用机制。 方法 对接种肝癌敏感细胞BEL-7402和肝癌耐药细胞BEL-7402/5-FU的移植瘤裸鼠,通过索拉菲尼、CII-3和脱脂膏药物处理后,观察给药后裸鼠血清生化指标,裸鼠瘤块变化和心、肝、肾、脾等脏器指数的变化,以及肝脏和瘤块病理学改变,同时观察肿瘤组织细胞中的自噬相关因子和侵袭转移相关因子的变化。 结果 CII-3和脱脂膏能使裸鼠体重呈下降趋势,并抑制肿瘤的生长,明显减轻瘤重(P < 0.01);CII-3能提高脾脏指数和心脏指数(P < 0.05);与耐药组相比,CII-3组裸鼠血清CR有升高趋势(P < 0.05);索拉菲尼和CII-3处理组能诱导肿瘤组织坏死,作用优于脱脂膏组;与耐药组相比,除AKT外(P > 0.05),CII-3和脱脂膏给药组均能明显抑制自噬和侵袭转移相关因子(P < 0.05)。 结论 美洲大蠊提取物CII-3和脱脂膏均能抑制肝癌耐药细胞的自噬和侵袭转移,同时具有较好的抑瘤作用。其对裸鼠肝癌移植瘤的肝肾损伤有待进一步观察。 Abstract:Objective To investigate the effects and preliminary mechanisms of the extract CII-3 and defatted ointment of Periplaneta americana on autophagy, invasion and metastasis of drug-resistant hepatocellular carcinoma cell line BEL-7402/5-FU. Methods The transplantation tumors of liver cancer-sensitive cells BEL-7402 and liver cancer-resistant cells BEL-7402/5-FU were treated with Sorafenib, CII-3, and defatted ointment drugs. Then, the serum biochemical indicators of the nude mice, changes in tumor size, and changes in organ indices such as heart, liver, kidney, and spleen were observed after drug administration. The histopathological changes in the liver and tumor were also observed, along with changes in autophagy-related factors and invasion and metastasis-related factors in tumor tissue cells. Results CII-3 and the deffated cream reduced the body weight of nude mice and inhibited tumor growth, significantly reducing tumor weight (P < 0.01). CII-3 increased the spleen index and heart index (P < 0.05); compared to the drug-resistant group, the CII-3 group showed an upward trend in serum CR (P < 0.05). Sorafenib and CII-3 treatment induced tumor tissue necrosis, with better effects than the deffatede ointment group; compared to the drug-resistant group, both the CII-3 and deffatede ointment groups significantly inhibited autophagy and invasion-related factors, except for AKT (P < 0.05). Conclusion Periplaneta americana extract CII-3 and deffatede ointment can inhibit the autophagy, invasion and metastasis of drug-resistant hepatocellular carcinoma cells, and also have a good anti-tumor effect. Further observation is needed for its liver and kidney damage on nude mice with hepatocellular carcinoma transplanted tumors. -
图 1 CII-3和脱脂膏对皮下移植瘤裸鼠体重、肿瘤体积及瘤重的影响
A:CII-3和脱脂膏对皮下移植瘤裸鼠体重的影响;B:CII-3和脱脂膏对皮下移植瘤裸鼠瘤体积的影响;C:CII-3和脱脂膏对皮下移植瘤裸鼠瘤重的影响。与敏感组相比较,*P < 0.05, **P < 0.01;与耐药组比较,#P < 0.05, ##P < 0.01;与索拉非尼组相比较,△P < 0.05,△△P < 0.01。
Figure 1. Effects of CII-3 and defatted ointment on body weight,tumor volume and tumor weight of nude mice with subcutaneously transplanted tumor
表 1 自噬相关因子引物序列表
Table 1. Primers sequence of autophagy related factors
名称 Forward Reverse 鼠ATG5 TGTGCTTCGAGATGTGTGGTT ACCAACGTCAAATAGCTGACTC ATG7 TGACCTTCGCGGACCTAAAGA CCCGGATTAGAGGGATGCTC 鼠BECLIN AGGCGAAACCAGGAGAGAC CCTCCCCGATCAGAGTGAA 鼠PIK3C3 AACAACCGTGTCGCTCTTTG GAACCATCTGCCTCCACGTTA 鼠LC3B GTCCTGGACAAGACCAAGTTCC CCATTCACCAGGAGGAAGAAGG 鼠P62 CCGGCTGATTGAGTCCCTC CCCCGATGTCGTAATTCTTGG GAPDH AGGTCGGTGTGAACGGATTTG TGTAGACCATGTAGTTGAGGTCA 表 2 侵袭转移相关因子引物序列表
Table 2. Primer sequence of invasion and metastasis related factors
名称 Forward Reverse 鼠MMP2 CAAGGATGGACTCCTGGCACAT TACTCGCCATCAGCGTTCCCAT 鼠MMP9 GCTGACTACGATAAGGACGGCA TAGTGGTGCAGGCAGAGTAGGA 鼠mTOR GGCACACATTTGAAGAAGCAG CTCGTTGAGGATCAGCAAGG 鼠AKT CCTTTATTGGCTACAAGGAACGG GAAGGTGCGCTCAATGACTG 鼠4EBP1 GGGGACTACAGCACCACTC GTTCCGACACTCCATCAGAAAT GAPDH TGGCCTTCGTGTTCCTAC GAGTTGTGTTGAAGTCGCA 表 3 CII-3和脱脂膏对皮下移植瘤裸鼠脏器指数的影响(
$\bar x \pm s $ ,n = 12)Table 3. Effects of CII-3 and defatted ointment on organ index in nude mice with subcutaneously transplanted tumor (
$\bar x \pm s $ ,n = 12)组别 心脏指数(mg/g) 肝脏指数(mg/g) 脾脏指数(mg/g) 肺脏指数(mg/g) 肾脏指数(mg/g) 空白组 6.02 ± 0.80 56.73 ± 7.30 6.02 ± 0.80 9.03 ± 1.60 17.84 ± 2.10 敏感组 5.98 ± 0.90 60.50 ± 8.89 5.98 ± 0.90 9.12 ± 2.12 18.16 ± 1.17 耐药组 6.67 ± 1.55 63.74 ± 8.09 6.67 ± 1.55 9.68 ± 2.79 17.21 ± 3.17 索拉非尼组 6.67 ± 1.01 69.42 ± 8.81▲*# 6.67 ± 1.01 11.43 ± 3.52 17.45 ± 4.59 CII-3组 7.12 ± 1.55▲*# 58.14 ± 10.6△ 7.12 ± 1.55▲* 11.54 ± 2.97 18.06 ± 3.54 脱脂膏组 6.08 ± 0.71 52.67 ± 8.10#△ 6.08 ± 0.71 10.37 ± 3.14 20.85 ± 3.38△ F 3.45 9.31 3.45 3.31 3.53 P 0.021 < 0.01 0.021 0.025 0.019 与空白组比较,▲P < 0.05,▲▲P < 0.01;与敏感组比较,*P < 0.05,**P < 0.01;与耐药组比较,#P < 0.05,##P < 0.01;与索拉非尼组比较,△P < 0.05,△△P < 0.01。 表 4 CII-3和脱脂膏对皮下移植瘤裸鼠生化指标的影响(
$\bar x \pm s $ ,n = 12)Table 4. Effects of CII-3 and defatted ointment on biochemical indexes of nude mice with subcutaneously transplanted tumor (
$\bar x \pm s $ ,n = 12)组别 AST(U/L) ALT(U/L) CR(μmol/L) BUN(mmol/L) 空白组 481.22 ± 62.98 13.99 ± 2.00 19.61 ± 2.75 5.34 ± 0.41 敏感组 660.91 ± 210.46▲ 12.27 ± 0.53 13.95 ± 4.25▲ 3.90 ± 0.51 耐药组 500.51 ± 83.87* 21.91 ± 5.26▲** 14.93 ± 2.35 4.88 ± 0.97 索拉非尼组 498.53 ± 140.13* 8.42 ± 2.34▲## 22.66 ± 4.13*# 4.83 ± 1.80 CII-3组 656.83 ± 106.84▲#△ 17.93 ± 1.88**△ 24.88 ± 4.59▲*# 3.33 ± 0.54▲#△ 脱脂膏组 491.06 ± 62.76# 12.81 ± 1.83# 19.92 ± 7.61* 4.64 ± 1.13 F 10.13 62.34 17.62 10.83 P < 0.01 <0.01 <0.01 <0.01 与空白组比较,▲P < 0.05,▲▲P < 0.01;与敏感组比较,*P < 0.05,**P < 0.01;与耐药组比较,#P< 0.05,##P < 0.01;与索拉非尼组比较,△P < 0.05,△△P < 0.01。 表 5 CII-3和脱脂膏对移植瘤裸鼠自噬相关因子mRNA的影响 (
$\bar x \pm s $ ,n = 12)Table 5. Effects of CII-3 and defatted ointment on autophagy related factor mRNA in nude mice with transplanted tumor (
$\bar x \pm s $ ,n = 12)组别 ATG5 ATG7 BECLIN LC3B PIK3C3 P62 敏感组 0.08 ± 0.01 0.04 ± 0.01 0.05 ± 0.03 0.03 ± 0.02 0.03 ± 0.00 0.04 ± 0.01 耐药组 1.00 ± 0.00** 1.00 ± 0.00** 1.00 ± 0.00** 1.00 ± 0.00** 1.00 ± 0.00** 1.00 ± 0.00** 索拉菲尼组 0.30 ± 0.14*# 0.03 ± 0.00## 0.02 ± 0.01## 0.16 ± 0.04**## 0.10 ± 0.02**## 0.1 ± 0.01**## CII-3组 0.16 ± 0.05## 0.05 ± 0.02## 0.12 ± 0.05*##△ 0.13 ± 0.06**## 0.04 ± 0.01##△ 0.08 ± 0.02*## 脱脂膏组 0.27 ± 0.12*## 0.07 ± 0.02## 0.12 ± 0.06*##△ 0.33 ± 0.01**##△△ 0.14 ± 0.03**##△ 0.24 ± 0.06**##△△ F 300.26 16442.05 1974.64 2171.41 10020.07 3148.02 P < 0.01 < 0.01 < 0.01 < 0.01 < 0.01 < 0.01 与敏感组比较,*P < 0.05,**P < 0.01;与耐药组比较,#P < 0.05,##P < 0.01;与索拉非尼组比较,△P < 0.05,△△P < 0.01。 表 6 CII-3和脱脂膏对移植瘤裸鼠侵袭转移相关因子mRNA的影响(
$\bar x \pm s $ ,n = 12)Table 6. Effects of CII-3 and defatted ointment on the mRNA of invasion and metastasis related factors in nude mice with transplanted tumor (
$\bar x \pm s $ ,n = 12)组别 MMP2 MMP9 mTOR AKT 4EBP1 敏感组 0.01 ± 0.00 0.01 ± 0.02 0.04 ± 0.01 0.02 ± 0.01 0.23 ± 0.08 耐药组 1.00 ± 0.00** 1.00 ± 0.00* 1.00 ± 0.00** 1.00 ± 0.00 1.00 ± 0.00** 索拉非尼组 0.02 ± 0.01**## 0.18 ± 0.08# 0.04 ± 0.01## 0.11 ± 0.06 1.80 ± 0.30**## CII-3组 0.01 ± 0.01## 0.00 ± 0.00# 0.08 ± 0.02## 0.17 ± 0.10 0.15 ± 0.06# 脱脂膏组 0.04 ± 0.02## 0.03 ± 0.01# 0.15 ± 0.30**##△ 0.20 ± 0.32 0.33 ± 0.15# F 26096.68 2171.29 154.83 156.90 325.86 P < 0.01 < 0.01 < 0.01 < 0.01 < 0.01 与敏感组比较,*P < 0.05,**P < 0.01;与耐药组比较,#P < 0.05,##P < 0.01;与索拉非尼组比较,△P < 0.05,△△P < 0.01。 -
[1] 曹毛毛,李贺,孙殿钦,等. 全球肝癌2020年流行病学现状[J]. 中华肿瘤防治杂志,2022,29(5):322-328. [2] 中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗指南(2022年版)[J]. 中华肝脏病杂志,2022,30(4):367-388. doi: 10.3760/cma.j.cn501113-20220413-00193 [3] Yu Y,Wang Y,Xiao X,et al. MiR-204 inhibits hepatocellular cancer drug resistance and metastasis through targeting NUAK1[J]. Biochemistry and Cell Biology,2019,97(5):563-570. doi: 10.1139/bcb-2018-0354 [4] Zhang DY,Wang W,Sun XJ,et al. AMPK regulates autophagy by phosphorylating BECLIN at threonine 388[J]. Autophagy,2016,12(9):1447-1459. doi: 10.1080/15548627.2016.1185576 [5] 何晓晓,熊枝繁,邱梦君,等. 阿帕替尼对人肝癌裸鼠皮下移植瘤生长及生物钟基因表达的影响[J]. 山东医药,2018,58(8):31-33. doi: 10.3969/j.issn.1002-266X.2018.08.008 [6] 李银蕊,吕鸿,彭芳,等. 美洲大蠊多肽PAE2逆转肝癌多药耐药性[J]. 中国实验方剂学杂志,2021,27(5):52-61. [7] 尤金炜,张立波,方天,等. 绿色荧光裸鼠血液生理生化指标检测分析[J]. 中国比较医学杂志,2013,23(9):23-26. doi: 10.3969/j.issn.1671.7856.2013.009.005 [8] 吕鸿,王瑶,张蕊,等. 美洲大蠊多肽PAP-2对H22荷瘤小鼠的抑瘤作用研究[J]. 中国药房,2019,30(7):927-931. [9] 谭耀红. 肿瘤多药耐药机制及抗耐药肿瘤新药的研究[D]. 北京: 中国协和医科大学, 2003. [10] 田雨弘,高鸽,刘颖,等. 桔梗茎叶皂苷对H22荷瘤小鼠的抗肿瘤作用及机制研究[J]. 毒理学杂志,2016,30(1):45-48. [11] 王彦权,张鸿翰,吕鸿,等. 美洲大蠊多肽PAE2逆转耐药细胞株多药耐药性的研究[J]. 大理大学学报,2021,6(8):1-6. doi: 10.3969/j.issn.2096-2266.2021.08.001 [12] 詹东梅,刘延庆. 中药逆转胃癌多药耐药的研究进展[J]. 癌症进展,2020,18(13):1307-1311. [13] Zhao Y,Yang A,Tu P,et al. Anti-tumor effects of the American cockroach,Periplaneta americana[J]. Chin Med,2017,12:26. doi: 10.1186/s13020-017-0149-6 [14] Kim D G,Jung K H,Lee D G,et al. 20(S)-Ginsenoside Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular carcinoma to doxorubicin[J]. Oncotarget,2014,5(12):4438-4451. doi: 10.18632/oncotarget.2034 [15] Hashemi M,Nadafzadeh N,Imani M H,et al. Targeting and regulation of autophagy in hepatocellular carcinoma: Revisiting the molecular interactions and mechanisms for new therapy approaches[J]. Cell Commun Signal,2023,21(1):32. doi: 10.1186/s12964-023-01053-z [16] 陈怡璇,王琳,夏秀宏,等. 自噬在肝细胞癌中的作用机制研究进展[J]. 昆明医科大学学报,2023,44(4):159-164. doi: 10.12259/j.issn.2095-610X.S20230416 [17] Han Z,Liu D,Chen L,et al. PNO1 regulates autophagy and apoptosis of hepatocellular carcinoma via the MAPK signaling pathway[J]. Cell Death Dis,2021,12(6):552. doi: 10.1038/s41419-021-03837-y [18] Zhang F,Gao J,Liu X,et al. LATS-regulated nuclear-cytoplasmic translocation of SREBP2 inhibits hepatocellular carcinoma cell migration and invasion via epithelial-mesenchymal transition[J]. Mol Carcinog,2023,62(7):963-974. doi: 10.1002/mc.23538 [19] 于珊珊,张华,张婷婷,等. 美洲大蠊药理作用及临床应用研究[J]. 辽宁中医药大学学报,2016,18(4):228-231. doi: 10.13194/j.issn.1673-842x.2016.04.072 [20] 李彩琳,吴定宇,吕鸿,等. 美洲大蠊提取物逆转人肝癌细胞HepG2/ADM多药耐药的机制研究[J]. 中国药房,2020,31(15):1816-1823. doi: 10.6039/j.issn.1001-0408.2020.15.05 [21] Xiao Y, Gao C, Wu J, et al. Periplaneta americana extract alleviates steatohepatitis in a mouse model by modulating HMGB1-mediated inflammatory response[J]. Front Pharmacol, 2022, 13(期?): 995523.Xiao Y,Gao C,Wu J,et al. Periplaneta americana extract alleviates steatohepatitis in a mouse model by modulating HMGB1-mediated inflammatory response[J]. Front Pharmacol,2022,13:995523. [22] Shi W,An L,Zhang J,et al. Periplaneta americana extract ameliorates lipopolysaccharide-induced liver injury by improving mitochondrial dysfunction via the AMPK/PGC-1α signaling pathway[J]. Exp Ther Med,2021,22(4):1138. doi: 10.3892/etm.2021.10572 [23] Huang F,Wang B R,Wang Y G. Role of autophagy in tumorigenesis,metastasis,targeted therapy and drug resistance of hepatocellular carcinoma[J]. World J Gastroenterol,2018,24(41):4643-4651. doi: 10.3748/wjg.v24.i41.4643 [24] Wang J,Zhu Y,Ai X,et al. Long noncoding RNA 02027 inhibits proliferation,migration and invasion of hepatocellular carcinoma via miR-625-3p/PDLIM5 pathway[J]. J Gene Med,2023,25(6):e3485. doi: 10.1002/jgm.3485 [25] Zeng J,Liu W,Fan Y Z,et al. PrLZ increases prostate cancer docetaxel resistance by inhibiting LKB1/AMPK-mediated autophagy[J]. Theranostics,2018,8(1):109-123. doi: 10.7150/thno.20356 [26] Ong C W M,Elkington P T,Brilha S,et al. Neutrophil-derived MMP-8 drives AMPK-dependent matrix destruction in human puLmonary tuberculosis[J]. PloS Pathog,2015,11(5):e1004917. doi: 10.1371/journal.ppat.1004917 -






 下载:
下载: