Butyric Acid Protects Intestinal Epithelial Barrier from Injury Induced by TNFα
-
摘要:
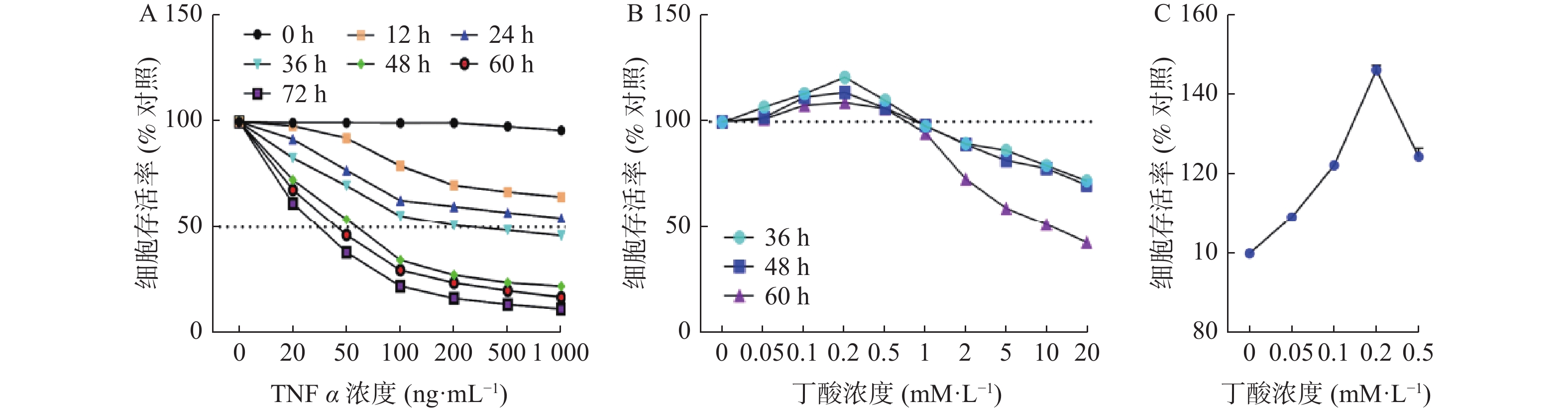
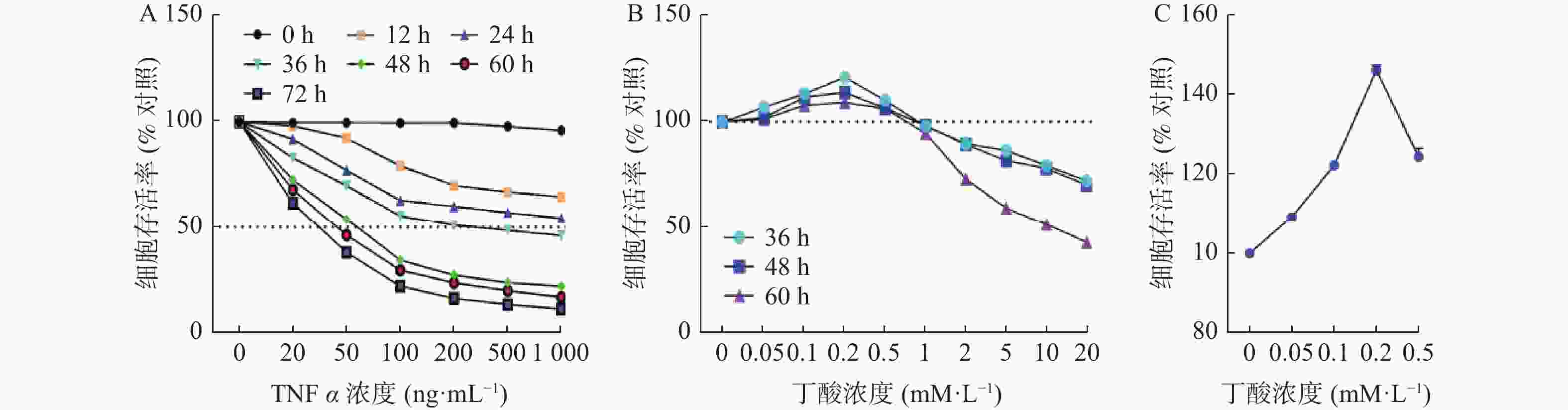
目的 探讨丁酸对TNFα所致肠上皮屏障损伤的保护作用。 方法 用CCK-8细胞活力检测探索TNFα对Caco2发挥损伤作用的最佳浓度和时间,并以此为基础探索在TNFα发挥损伤作用最佳作用时间附近丁酸对Caco2细胞的保护情况,随后探索TNFα和丁酸共同作用于Caco2细胞时的最佳时间和浓度,并检测Caco2细胞单层上皮屏障的FITC-dextran渗透率,紧密连接ZO-1和Occludin的mRNA表达情况及免疫荧光观察TNFα和丁酸共同作用后细胞的生长情况及ZO-1和Occludin在Caco2中的表达和分布。 结果 100 ng/mL的TNFα刺激48 h能明显降低Caco2的细胞存活率(P < 0.0001);丁酸作用于Caco2细胞48 h,0.2 mM/L的丁酸能明显提高Caco2的细胞存活率(P < 0.0001)。当TNFα和丁酸共同作用时,与TNFα干预组相比,丁酸可以明显降低TNFα所致的肠上皮单层屏障的FITC-dextran渗透率(P < 0.0001),提高ZO-1(P < 0.01)和Occludin(P < 0.01)的表达及稳定其在Caco2细胞中的分布。 结论 丁酸可以减轻TNFα所致的肠上皮屏障损伤,为进一步明确丁酸在溃疡性结肠炎治疗中作用机制提供实验基础。 Abstract:Objective To explore the protective effect of butyric acid on intestinal epithelial barrier damage induced by TNF α. Methods CCK-8 cell viability assay was used to determine the optimal concentration and time of TNFα in exerting damaged effects on Caco2 damage, and based on this, the protective effect of butyric acid on Caco2 cells was explored around the optimal time when TNFα exerted damage. Subsequently, the optimal time and concentration of TNFα and butyric acid acting on Caco2 cells were explored, and the FITC-dextran permeability of the Caco2 cell monolayer epithelial barrier, the mRNA expression of ZO-1 and Occludin, and the growth of cells after TNFα and butyric acid co-treatment were detected, as well as the expression and distribution of ZO-1 and Occludin in Caco2 cells were observed by immunofluorescence. Results TNFα, at a concentration of 100 ng/mL significantly decreased the cell viability of Caco2 after 48 hours of stimulation (P < 0.0001); butyrate at a concentration of 0.2mM/L significantly increased the cell viability of Caco2 after 48 hours of treatment (P < 0.0001). When TNFα and butyrate were co-administered, compared with the TNFα intervention group, butyrate significantly decreased the FITC-dextran permeability of the intestinal epithelial monolayer barrier caused by TNFα (P < 0.0001), and increased the expression of ZO-1 (P < 0.01) and Occludin (P < 0.01), and stabilized their distribution in Caco2 cells. Conclusion Butyrate can alleviate TNFα-induced damage to intestinal epithelial barrier, providing experimental basis for further elucidating the mechanism of butyrate in the treatment of ulcerative colitis. -
Key words:
- Caco2 /
- Ulcerative colitis /
- TNF α /
- Butyric acid /
- ZO-1 /
- Occludin
-
图 4 TNFα和丁酸单独或共同作用48 h对Caco2细胞紧密连接蛋白的mRNA表达的影响
A:TNFα(100 ng/mL)和丁酸(0.2 mM/L)单独或共同作用48 h对Caco2细胞ZO-1的mRNA表达的影响;B:TNFα(100 ng/mL)和丁酸(0.2 mM/L)单独或共同作用48 h对Caco2细胞Occludin的mRNA表达的影响;ns P > 0.05,* P < 0.05,** P < 0.01,***P < 0.001。
Figure 4. The mRNA expression of tight junction proteins in Caco2 cells after exposure to TNF α or butyric acid for 48 h
图 5 TNFα和丁酸和作用48h后Caco2细胞ZO-1表达和分布的变化(免疫荧光染色,放大倍数为×800,DAPI标记细胞核为蓝色,ZO-1染为绿色)
A:对照组;B:丁酸干预组;C:TNFα和丁酸干预组;D:TNFα干预组
Figure 5. The expression and distribution of ZO-1 on Caco2 cells after exposure to TNF α and Butyric acid for 48 hours (immunofluorescence staining,magnification at ×800 ,DAPI labeled nucleus as blue,ZO-1 stained as green)
图 6 丁酸和TNFα作用48 h后Caco2细胞Occludin表达和分布的变化(免疫荧光染色,放大倍数为800倍,DAPI标记细胞核为蓝色,Occludin染为绿色)
A:对照组;B:丁酸干预组;C:TNFα和丁酸干预组;D:TNFα干预组
Figure 6. The expression and distribution of Occludin on Caco2 cells after exposure to TNF α and Butyric acid for 48 hours (immunofluorescence staining,magnification at ×800 ,DAPI labeled nucleus as blue,Occludin stained as green)
-
[1] Zhang W,Lyu M,Bessman N J,et al. Gut-innervating nociceptors regulate the intestinal microbiota to promote tissue protection[J]. Cell,2022,185(22):4170-4189.e20. doi: 10.1016/j.cell.2022.09.008 [2] Tan G,Huang C,Chen J,et al. An IRF1-dependent pathway of TNFα-induced shedding in intestinal epithelial cells[J]. J Crohns Colitis,2022,16(1):133-142. doi: 10.1093/ecco-jcc/jjab134 [3] Abraham C,Abreu M T,Turner J R. Pattern recognition receptor signaling and cytokine networks in microbial defenses and regulation of intestinal barriers: Implications for inflammatory bowel disease[J]. Gastroenterology,2022,162(6):1602-1616.e6. doi: 10.1053/j.gastro.2021.12.288 [4] Nguyen N H,Solitano V,Vuyyuru S K,et al. Proactive therapeutic drug monitoring versus conventional management for inflammatory bowel diseases: A systematic review and meta-analysis[J]. Gastroenterology,2022,163(4):937-949.e2. doi: 10.1053/j.gastro.2022.06.052 [5] Matsuoka K,Kanai T. The gut microbiota and inflammatory bowel disease[J]. Semin Immunopathol,2015,37(1):47-55. doi: 10.1007/s00281-014-0454-4 [6] Nighot M,Al-Sadi R,Guo S,et al. Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by Toll-like receptor 4/Myeloid differentiation primary response 88 (MyD88) activation of myosin light chain kinase expression[J]. Am J Pathol,2017,187(12):2698-2710. doi: 10.1016/j.ajpath.2017.08.005 [7] Yang J,Pei G,Sun X,et al. RhoB affects colitis through modulating cell signaling and intestinal microbiome[J]. Microbiome,2022,10(1):149-170. doi: 10.1186/s40168-022-01347-3 [8] Foerster EG,Mukherjee T,Cabral-Fernandes L,et al. How autophagy controls the intestinal epithelial barrier[J]. Autophagy,2022,18(1):86-103. doi: 10.1080/15548627.2021.1909406 [9] Xiong T,Zheng X,Zhang K,et al. Ganluyin ameliorates DSS-induced ulcerative colitis by inhibiting the enteric-origin LPS/TLR4/NF-κB pathway[J]. J Ethnopharmacol,2022,289(8):115001-115014. [10] Kumar A,Priyamvada S,Ge Y,et al. A Novel Role of SLC26A3 in the maintenance of intestinal epithelial barrier Integrity[J]. Gastroenterology,2021,160(4):1240-1255.e3. doi: 10.1053/j.gastro.2020.11.008 [11] Guo H,Guo H,Xie Y,et al. Mo(3)Se(4) nanoparticle with ROS scavenging and multi-enzyme activity for the treatment of DSS-induced colitis in mice[J]. Redox Biol,2022,56(8):102441. [12] Wu X X,Huang X L,Chen R R,et al. Paeoniflorin prevents intestinal barrier disruption and inhibits lipopolysaccharide (LPS)induced inflammation in Caco2 cell monolayers[J]. Inflammation,2019,42(6):2215-2225. doi: 10.1007/s10753-019-01085-z [13] Beisner J,Filipe Rosa L,Kaden-Volynets V,et al. Prebiotic inulin and sodium butyrate attenuate obesity-induced intestinal barrier dysfunction by induction of antimicrobial peptides[J]. Front Immunol,2021,12:678360. doi: 10.3389/fimmu.2021.678360 [14] Kim JT,Napier DL,Kim J,et al. Ketogenesis alleviates TNFα-induced apoptosis and inflammatory responses in intestinal cells[J]. Free Radic Biol Med,2021,172:90-100. doi: 10.1016/j.freeradbiomed.2021.05.032 [15] Nakase H,Sato N,Mizuno N,et al. The influence of cytokines on the complex pathology of ulcerative colitis[J]. Autoimmun Rev,2022,21(3):103017. doi: 10.1016/j.autrev.2021.103017 [16] Song G,Gan Q,Qi W,et al. Fructose stimulated colonic arginine and proline metabolism dysbiosis,altered microbiota and aggravated intestinal barrier dysfunction in DSS-induced colitis rats[J]. Nutrients,2023,15(3):782-798. doi: 10.3390/nu15030782 [17] Greuter T,Rieder F,Kucharzik T,et al. Emerging treatment options for extraintestinal manifestations in IBD[J]. Gut,2021,70(4):796-802. doi: 10.1136/gutjnl-2020-322129 [18] Li G,Lin J,Zhang C,et al. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease[J]. Gut Microbes,2021,13(1):1968257-1968275. doi: 10.1080/19490976.2021.1968257 [19] Gasaly N,Hermoso M A,Gotteland M. Butyrate and the fine-tuning of colonic homeostasis: Implication for inflammatory bowel diseases[J]. Int J Mol Sci,2021,22(6):3061-3078. doi: 10.3390/ijms22063061 [20] Benvenuti L,D'Antongiovanni V,Pellegrini C,et al. Dietary supplementation with the probiotic SF68 reinforces intestinal epithelial barrier in Obese mice by improving butyrate bioavailability[J]. Mol Nutr Food Res,2023,67(13):e2200442. doi: 10.1002/mnfr.202200442 [21] Facchin S,Vitulo N,Calgaro M,et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease[J]. Neurogastroenterol Motil,2020,32(10):e13914. [22] Jourova L,Satka S,Frybortova V,et al. Butyrate treatment of DSS-induced ulcerative colitis affects the hepatic drug metabolism in mice[J]. Front Pharmacol,2022,13:936013. doi: 10.3389/fphar.2022.936013 -





 下载:
下载: