miR-148a-3p Targeting SMURF2 in Regulating Osteogenic Differentiation and Enamel Development during In Vitro Tooth Organogenesis
-
摘要:
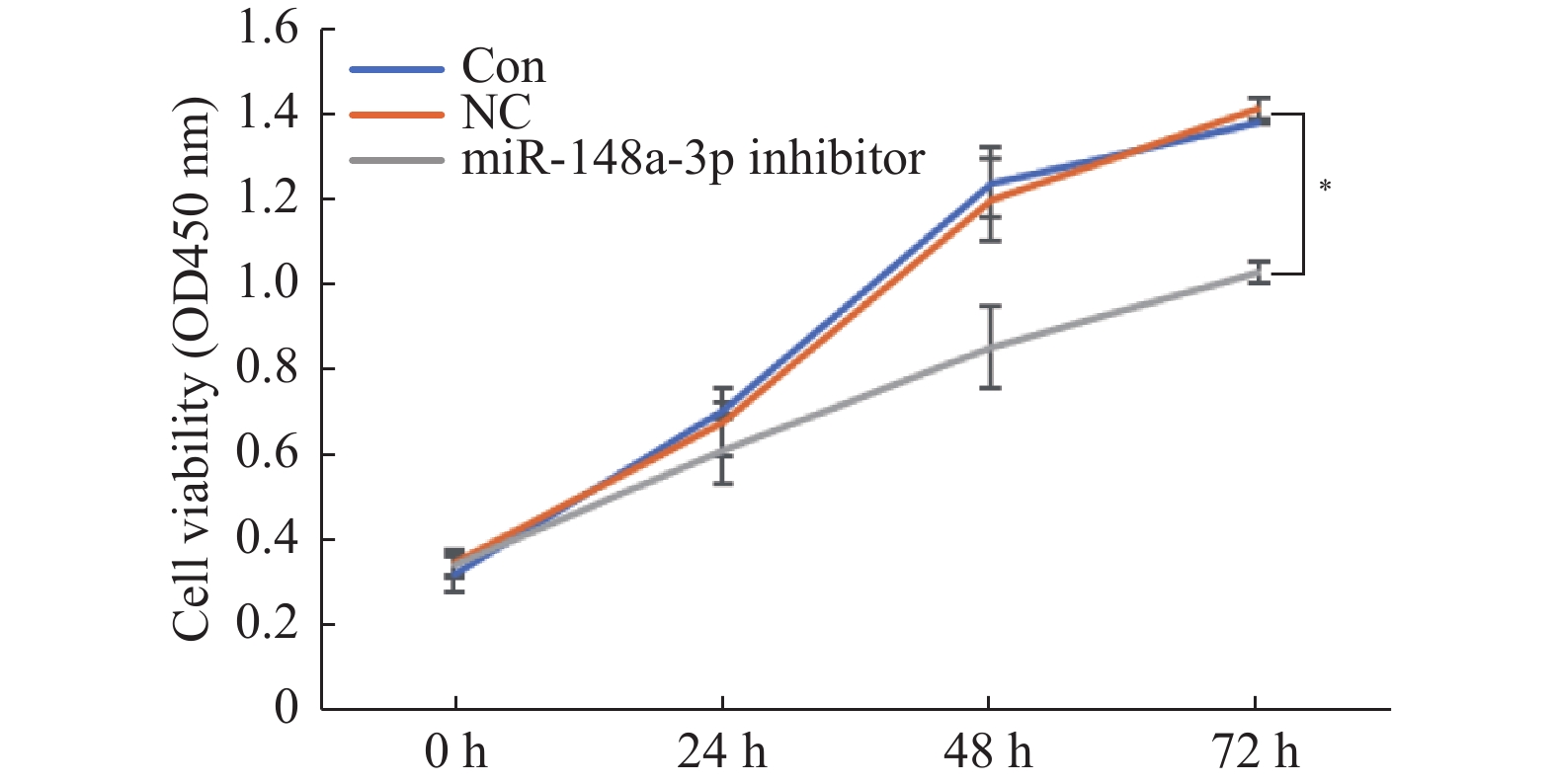
目的 探讨miR-148a-3p在体外牙齿发生中成骨分化和牙釉质发育中的作用,揭示其分子机制。 方法 获取人牙髓干细胞和口腔上皮细胞。将人牙髓干细胞转染miR-148a-3p mimics、miR-148a-3p抑制剂或阴性对照。通过在Matrigel基质凝胶上接种人牙髓干细胞并覆盖口腔上皮细胞,建立三维共培养系统。采用MTT实验评估miR-148a-3p对共培养细胞增殖和蛋白表达的影响,采用Western blotting实验检测成骨细胞分化(RUNX2)和牙釉质发育标志物(E-cadherin)蛋白表达水平,采用荧光素酶报告实验验证SMURF2(SMAD 特异性 E3泛素蛋白连接酶2)与miR-148a-3p的相互作用。 结果 在人牙髓干细胞中使用抑制剂下调miR-148a-3p后,3D共培养中的细胞活力降低(P < 0.05)。使用模拟物上调miR-148a-3p后,共培养中成骨标志物RUNX2和牙釉质发育标志物E-cadherin的表达增加 (P < 0.05)。TargetScan软件预测miR-148a-3p在SMURF2的3'-UTR中有结合位点。荧光素酶报告实验表明miR-148a-3p mimics抑制野生型载体的荧光素酶活性(P < 0.05),同时 Western blot 结果显示miR-148a-3p mimics下调SMURF2的表达(P < 0.05)。 结论 miR-148a-3p可能通过靶向SMURF2,调控RUNX2和E-cadherin的表达,参与了人牙髓干细胞成骨分化和上皮细胞牙釉质发育。为牙齿修复和治疗提供了潜在的治疗靶点。 -
关键词:
- miR-148a-3p /
- 牙源性间充质干细胞 /
- 成骨分化 /
- 牙釉质发育 /
- SMURF2
Abstract:Objective To explore the role of miR-148a-3p in osteogenic differentiation and enamel development during in vitro tooth organogenesis and uncover its underlying molecular mechanism. Methods Human dental pulp stem cells and oral epithelial cells were obtained. Human dental pulp stem cells were transfected with miR-148a-3p mimics, miR-148a-3p inhibitors, or a negative control. A three-dimensional co-culture system was established by seeding human dental pulp stem cells onto the Matrigel matrix and overlaying them with oral epithelial cells. The impact of miR-148a-3p on cell proliferation and protein expression was assessed using MTT assay. The expression levels of osteogenic marker RUNX2 and enamel development marker E-cadherin were determined through Western blotting. The interaction between SMURF2(SMAD-specific E3 ubiquitin-protein ligase 2) and miR-148a-3p was validated via a luciferase reporter assay. Results Downregulation of miR-148a-3p using its inhibitor in human dental pulp stem cells led to reduced cell viability in the 3D co-culture system(P < 0.05). Conversely, upregulation of miR-148a-3p using its mimic increased the expression of osteogenic marker RUNX2 and enamel development marker E-cadherin in the co-culture setting(P < 0.05). TargetScan software predicted a binding site for miR-148a-3p within the 3’ -UTR of SMURF2. Luciferase report Experiments showed that miR-148a-3p mimics significantly inhibited the luciferase activity of wild-type(P < 0.05), while Western blot results showed that miR-148a-3p mimics significantly down-regulated the expression of SMURF2(P < 0.05). Conclusion The research findings suggest that miR-148a-3p potentially regulates the expression of RUNX2 and E-cadherin by targeting SMURF2, thereby participating in osteogenic differentiation of human dental pulp stem cells and enamel development in oral epithelial cells during in vitro tooth organogenesis. This study provides promising therapeutic targets for dental repair and treatment. -
Key words:
- MiR-148a-3p /
- Dental pulp stem cells /
- Osteogenic differentiation /
- Enamel development /
- SMURF2
-
图 2 Western blotting法检测miR-148a-3p对RUNX2和N-cadherin蛋白表达水平的影响
A:RT-PCR检测miR-148a-3p表达水平;B:Western blotting法检测RUNX2和N-cadherin蛋白表达水平;C:灰度值分析RUNX2和N-cadherin蛋白相对表达量。与Con比较,*P < 0.05。
Figure 2. The effects of miR-148a-3p on the expression levels of RUNX2 and N-cadherin proteins were detected by Western blotting
-
[1] Morsczeck C. Dental stem cells for tooth regeneration: how far have we come and where next?[J]. Expert Opin Biol Ther,2023,23(6):527-537. doi: 10.1080/14712598.2023.2208268 [2] Wang T,Zhang X,Bikle D D. Osteogenic differentiation of periosteal cells during fracture healing[J]. J Cell Physiol,2017,232(5):913-921. doi: 10.1002/jcp.25641 [3] Shafaei H,Kalarestaghi H. Adipose-derived stem cells: An appropriate selection for osteogenic differentiation[J]. J Cell Physiol,2020,235(11):8371-8386. doi: 10.1002/jcp.29681 [4] Xiao L,Tsutsui T. Three-dimensional epithelial and mesenchymal cell co-cultures form early tooth epithelium invagination-like structures: expression patterns of relevant molecules[J]. J Cell Biochem,2012,113(6):1875-1885. doi: 10.1002/jcb.24056 [5] Brown C,McKee C,Bakshi S,et al. Mesenchymal stem cells: Cell therapy and regeneration potential[J]. J Tissue Eng Regen Med,2019,13(9):1738-1755. doi: 10.1002/term.2914 [6] Langenbach F,Handschel J. Effects of dexamethasone,ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro[J]. Stem Cell Res Ther,2013,4(5):117. doi: 10.1186/scrt328 [7] Zhang X,Zhou Y,Ye Y,et al. Human umbilical cord mesenchymal stem cell-derived exosomal microRNA-148a-3p inhibits neointimal hyperplasia by targeting Serpine1[J]. Arch Biochem Biophys,2022,719:109155. doi: 10.1016/j.abb.2022.109155 [8] Tian L,Zheng F,Li Z,et al. miR-148a-3p regulates adipocyte and osteoblast differentiation by targeting lysine-specific demethylase 6b[J]. Gene,2017,627:32-39. doi: 10.1016/j.gene.2017.06.002 [9] Sheng W, Jiang H, Yuan H, et al. miR-148a-3p facilitates osteogenic differentiation of fibroblasts in ankylosing spondylitis by activating the Wnt pathway and targeting DKK1. Exp Ther Med, 2022, 23(5): 365. [10] Zhang Y D, Chen Z, Song Y Q, et al. Making a tooth: growth factors, transcription factors, and stem cells. Cell Res, 2005, 15(5): 301-316. [11] 周雅川,周学东,郑黎薇. 微小RNAs在牙釉质发育过程中的表达和作用[J]. 华西口腔医学杂志,2017,35(3):328-333. doi: 10.7518/hxkq.2017.03.018 [12] Zhou Y,Zheng L,Sun J,et al. Expression and function of MicroRNAs in enamel development[J]. Curr Stem Cell Res Ther,2015,10(5):422-433. doi: 10.2174/1574888X10666150312101451 [13] Yin K,Paine M L. Bicarbonate transport during enamel maturation[J]. Calcif Tissue Int,2017,101(5):457-464. doi: 10.1007/s00223-017-0311-2 [14] Shi C,Huang F,Gu X,et al. Adipogenic miRNA and meta-signature miRNAs involved in human adipocyte differentiation and obesity[J]. Oncotarget,2016,7(26):40830-40845. doi: 10.18632/oncotarget.8518 [15] Sun Y, Xie L, Ren X, et al. miR-148a-3p regulates proliferation and apoptosis of idiopathic gingival fibroma by targeting NPTX1[J]. Oral Dis, 2023, https://doi.org/10.1111/odi.14655. [16] Khotib J,Marhaeny HD,Miatmoko A,et al. Differentiation of osteoblasts: The links between essential transcription factors[J]. J Biomol Struct Dyn,2023,41(19):10257-10276. [17] Li CY,Cha W,Luder HU,et al. E-cadherin regulates the behavior and fate of epithelial stem cells and their progeny in the mouse incisor[J]. Dev Biol,2012,366(2):357-366. doi: 10.1016/j.ydbio.2012.03.012 [18] Fan X,Jin S,Li Y,et al. Genetic and epigenetic regulation of E-cadherin signaling in human hepatocellular carcinoma[J]. Cancer Manag Res,2019,11:8947-8963. doi: 10.2147/CMAR.S225606 [19] Wang D,Zou Y,Huang X,et al. The role of SMURFs in non-cancerous diseases[J]. FASEB J,2023,37(8):e23110. doi: 10.1096/fj.202300598R [20] Schminke B,Kauffmann P,Schubert A,et al. SMURF1 and SMURF2 in progenitor cells from articular cartilage and meniscus during late-stage osteoarthritis[J]. Cartilage,2021,13(2_suppl):117S-128S. doi: 10.1177/1947603520967069 [21] Zhao C,Xu Z,Wang Z,et al. Role of tumor necrosis factor-α in epithelial-to-mesenchymal transition in transplanted kidney cells in recipients with chronic allograft dysfunction[J]. Gene,2018,642:483-490. doi: 10.1016/j.gene.2017.11.059 -






 下载:
下载: