Huashi Runjing Decoction Amelioretes the Inflammatory Response of Castrated Male Dry Eye Disease Rats by Regulating COX-2,TNF-α,and IL-6 Proteins
-
摘要:
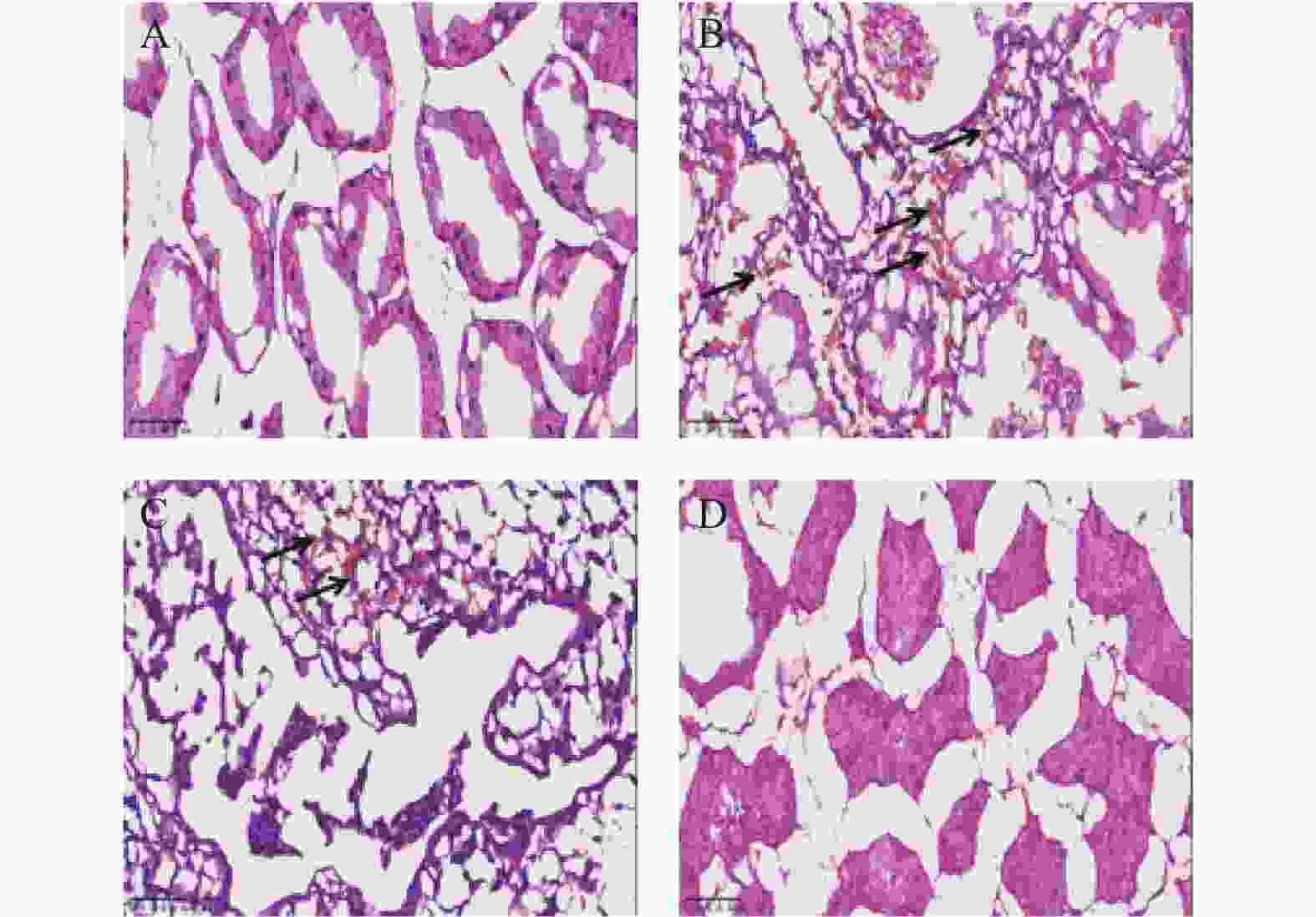
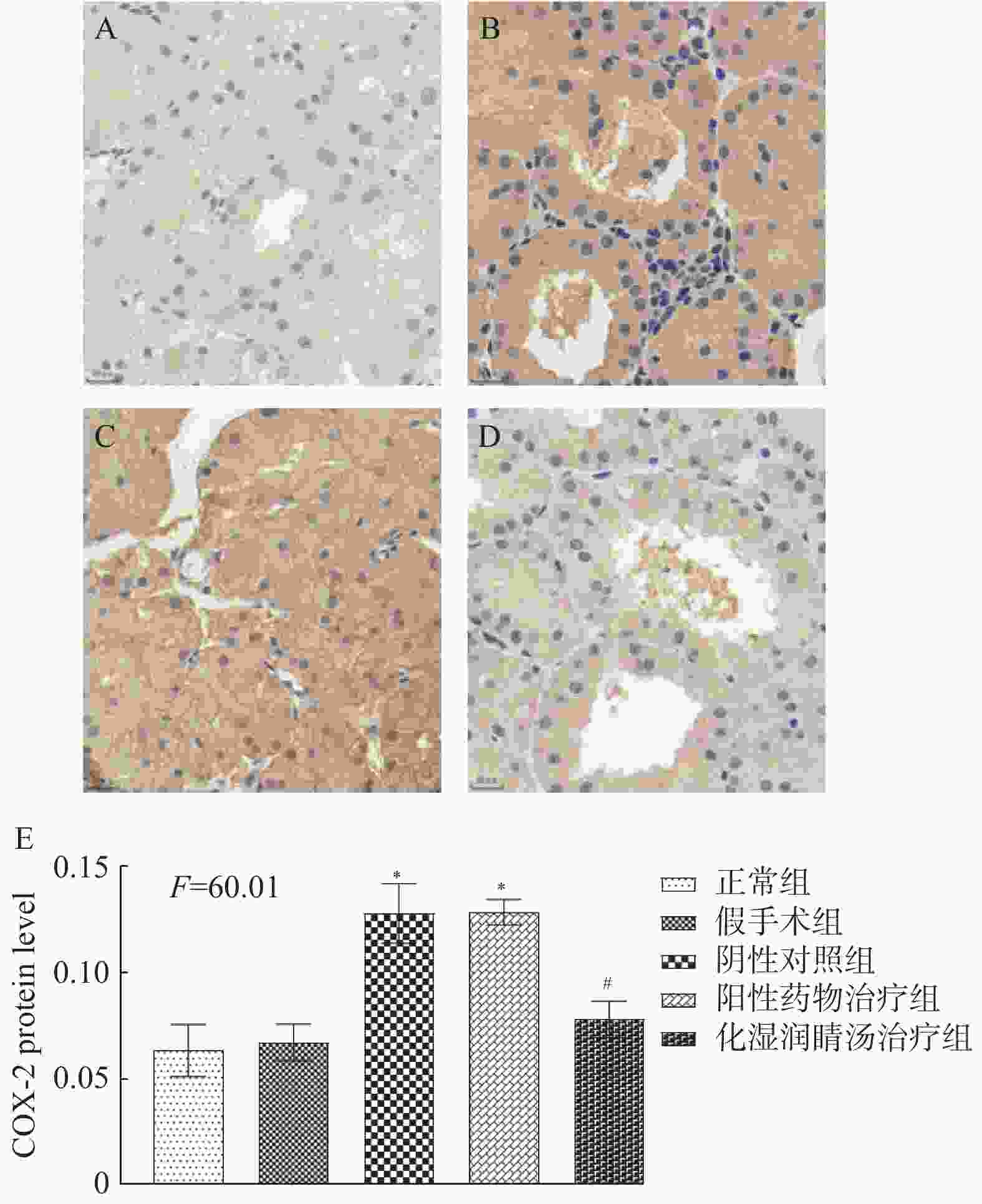
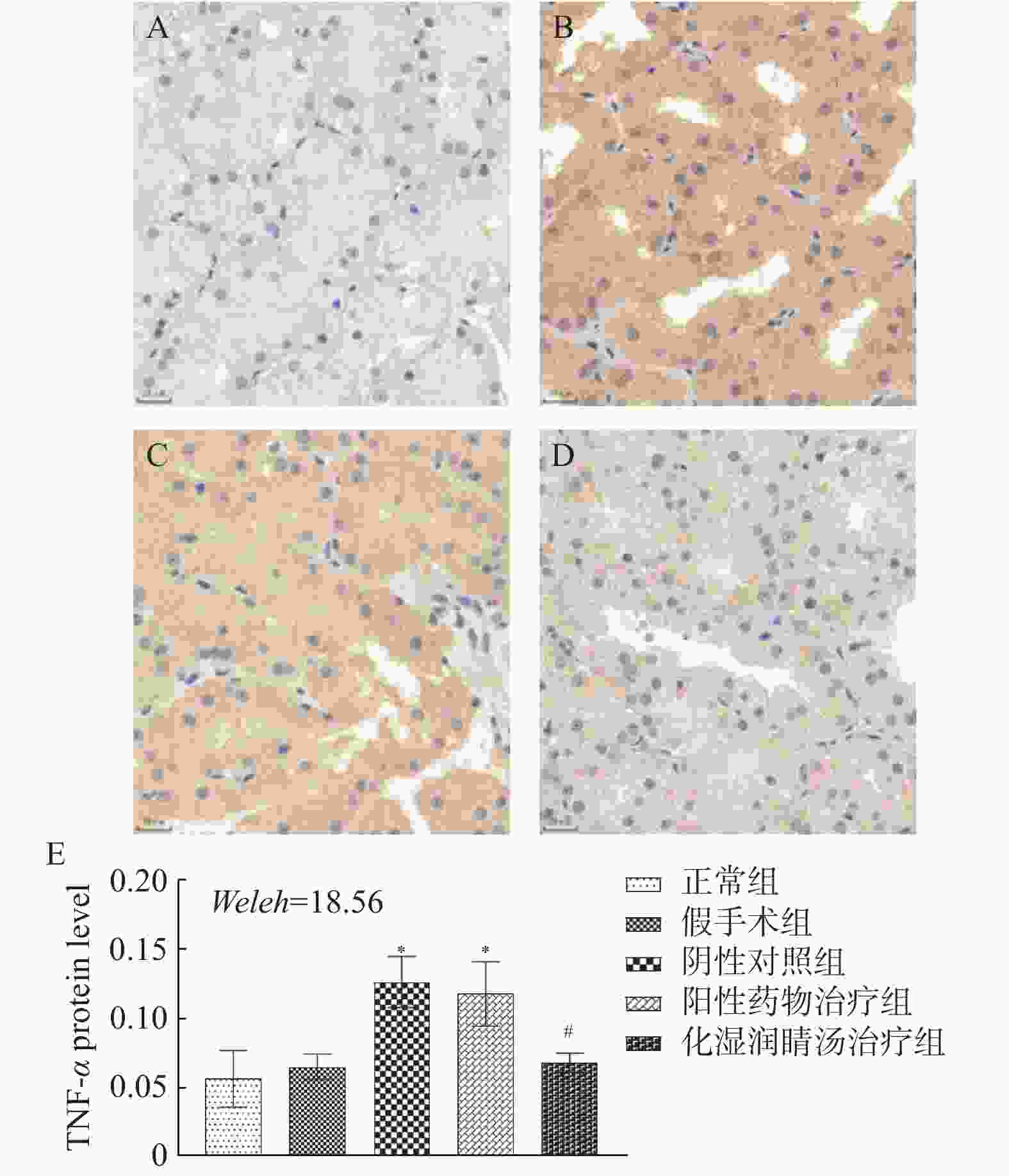
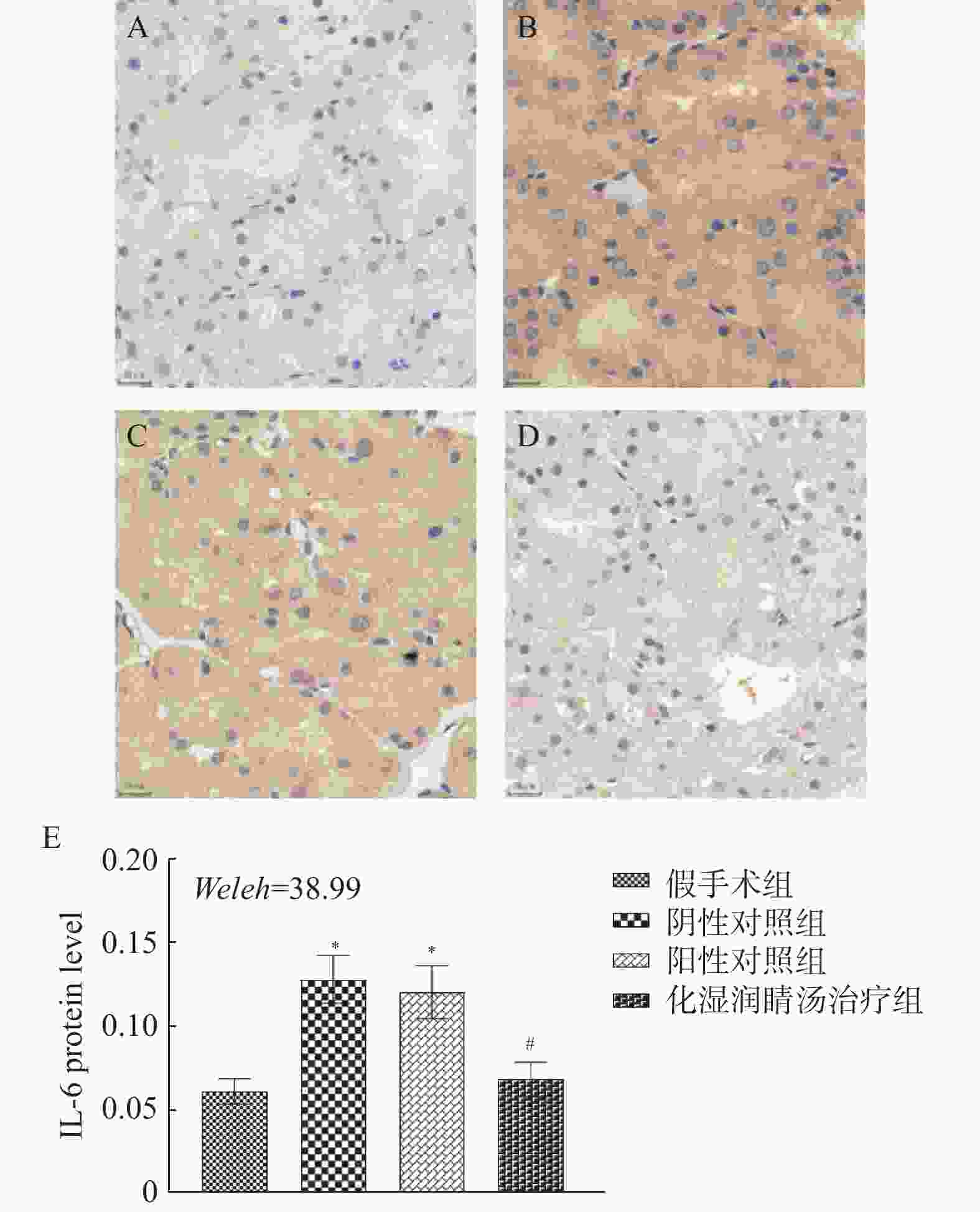
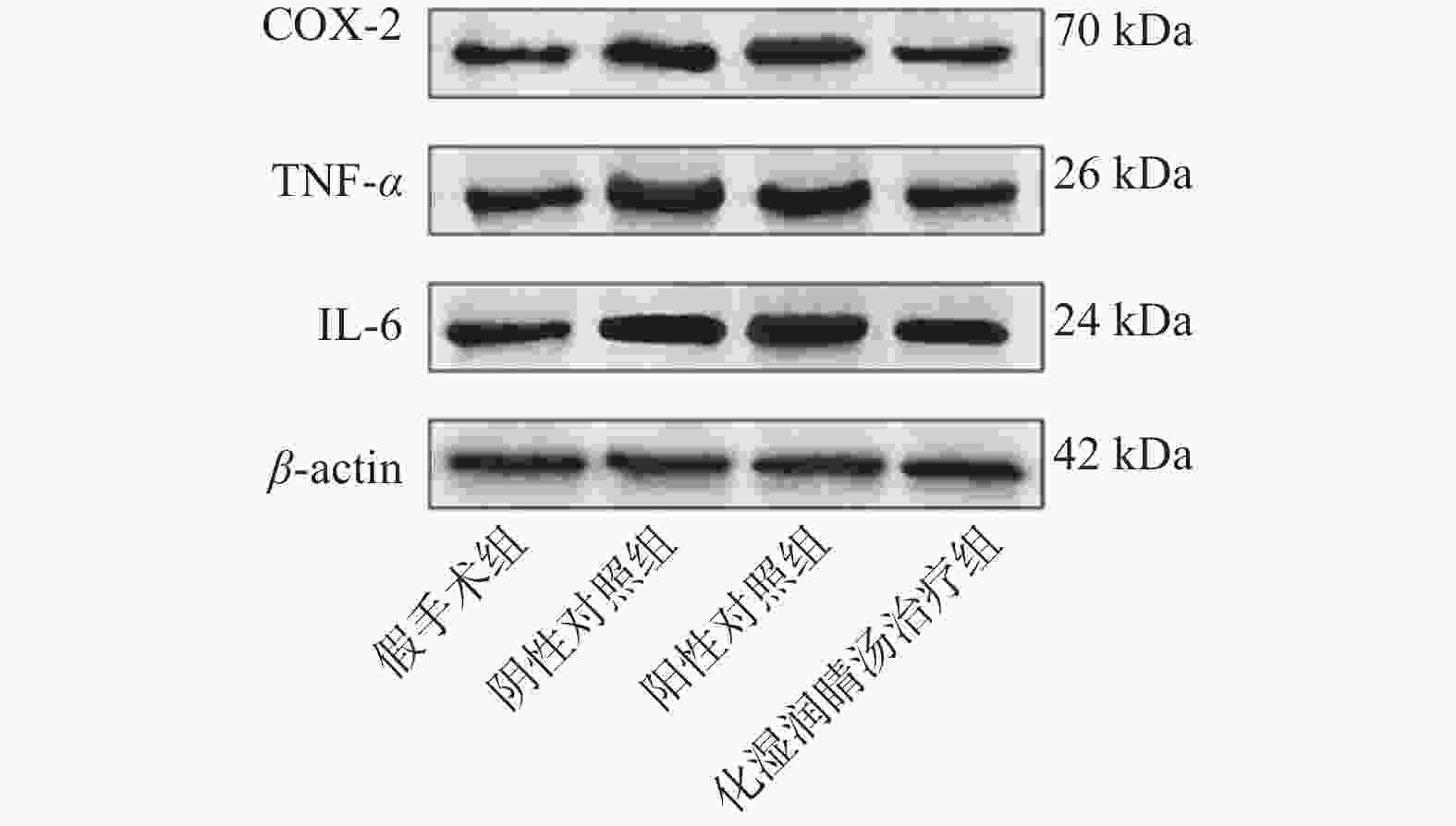
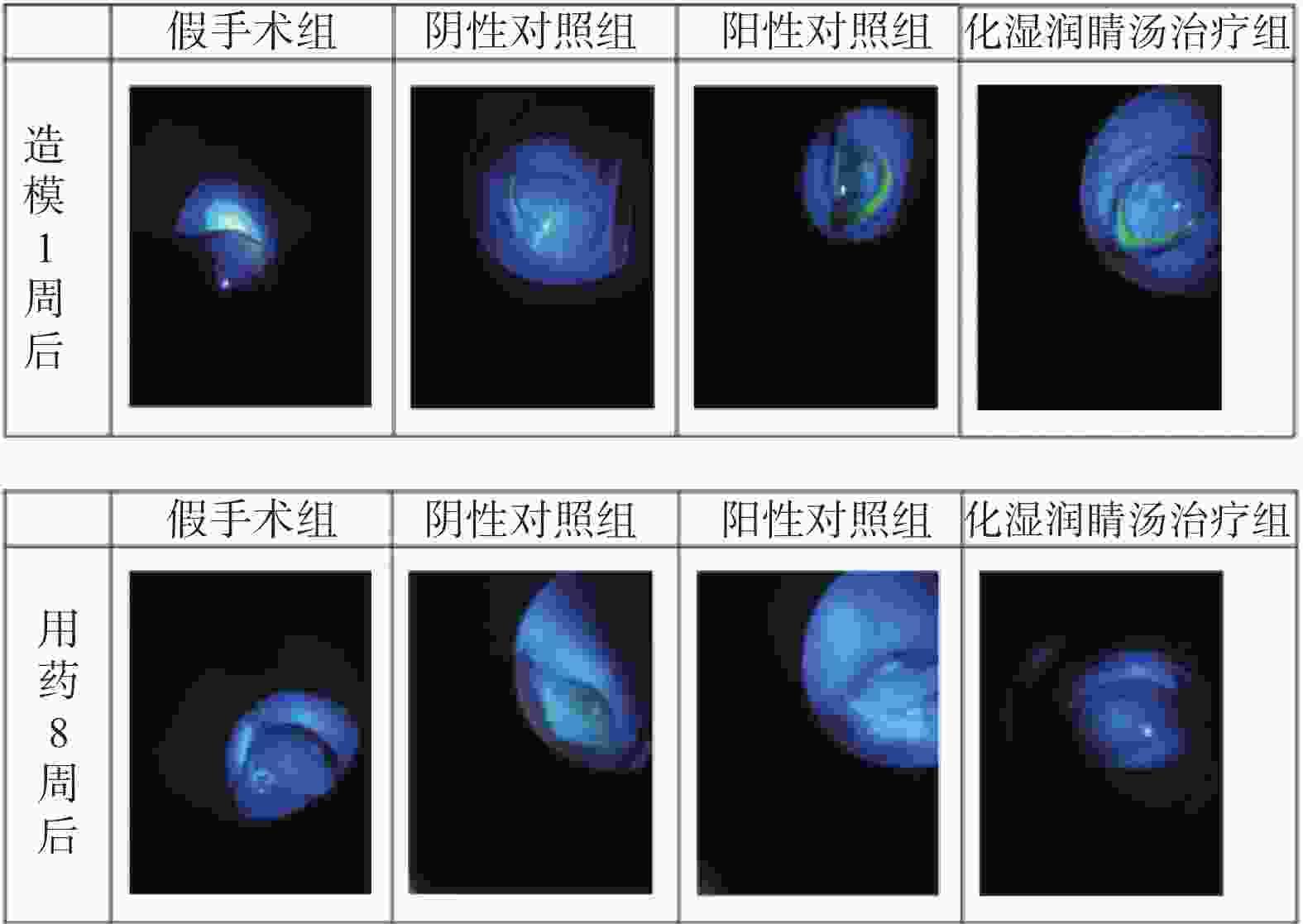
目的 观察化湿润睛汤是否能抑制去势雄性干眼病大鼠的炎症反应,并探讨其潜在的作用机制。 方法 将60只健康雄性SD大鼠随机分为4组,即假手术组15只、阴性对照组15只、阳性对照组15只、化湿润睛汤治疗组15只。假手术组大鼠只切开腹腔而不切除两侧睾丸及附睾;阴性对照组、阳性对照组、化湿润睛汤治疗组大鼠切开腹腔并切除两侧睾丸及附睾。建模1周后用角膜荧光素染色(fluorescein staining,FL)、泪膜破裂时间(break-up time,BUT)、泪液分泌试验(schirmer I-test,SIT)检测方法验证模型。待实验大鼠伤口基本愈合之后开始予以相应的药物干预。用药8周后分别采用FL法、BUT法和SIT法检测各组大鼠角膜破损程度、泪膜破裂时间和泪液分泌量;ELISA法观察各组大鼠血清中COX-2、TNF-α、IL-6 水平;HE染色观察各组大鼠泪腺组织中病理形态学改变情况;免疫组化和Western blot法观察各组大鼠泪腺组织中炎症因子COX-2、TNF-α和 IL-6蛋白表达的改变。 结果 与假手术组比较,阴性对照组、阳性对照组、化湿润睛汤治疗组大鼠角膜荧光素钠阳性染色面积增加,FL评分增高,泪膜破裂时间缩短以及泪液分泌量减少,血清COX-2、TNF-α、IL-6 水平升高,泪腺组织腺泡萎缩变小、形状不规则、结构紊乱、胞质内酶原颗粒减少,并见大量炎性细胞浸润,泪腺组织中炎症因子COX-2、TNF-α和 IL-6蛋白表达水平增加(P < 0.05);与阴性对照组比较,化湿润睛汤治疗组大鼠荧光素钠阳性染色面积减少、FL评分降低、泪膜破裂时间延长以及泪液分泌量增多,血清COX-2、TNF-α和IL-6水平降低,泪腺组织腺泡萎缩程度减轻,形状较为规则,结构较为清晰,胞质内酶原颗粒数量增加,炎性细胞浸润面积减少,泪腺组织中COX-2、TNF-α和IL-6蛋白表达水平降低(P < 0.05)。 结论 化湿润睛汤可降低去势雄性干眼病大鼠泪腺组织中炎症因子COX-2、TNF-α和 IL-6的蛋白表达,减轻其炎症反应。 Abstract:Objective To observe whether Huashi Runjing Decoction can inhibit the inflammatory response in rats with desmoplastic male dry eye disease, and to explore its potential mechanism of action. Methods 60 healthy male SD rats were randomly divided into 4 groups: sham surgery group of 15, negative control group of 15, positive control group of 15, Huashi Running Decoction group of 15). The sham operated group rats only underwent abdominal incision without removing both testicles and epididymis; Rats in the negative control group, positive control group and Huashi Runjing Decoction group were removed both testicles and epididymis. After 1 week of modeling, the model was validated by fluorescein staining(FL), break-up time(BUT), and tear secretion test(SIT). After the wound healed, the rats were given the appropriate drug interventions. The degree of corneal breakage, tear film rupture time and tear secretion of rats in each group were detected by FL method, BUT method and SIT method respectively after 8 weeks of drug administration. The levels of COX-2, TNF-α and IL-6 in the serum of each group of rats were observed by ELISA; HE staining was used to observe the pathomorphological changes in the lacrimal gland tissue of rats in each group. The expression of key inflammatory factors COX-2, TNF-α and IL-6 in the lacrimal gland tissues of rats in each group was observed by immunohistochemistry and Western blot. Results Compared with the sham-operated group, the negative control group, positive control group and Huashi Runjing Decoction group showed an increase in the area of sodium fluorescein positive staining in the cornea, a significant increase in FL score, a shortening of tear film rupture time and a decrease in tear secretion, an increase in serum COX-2, TNF-α and IL-6 levels, atrophy of the lacrimal gland tissue vesicles, an irregular shape, a disorganized structure, a decrease in intracytoplasmic zymogen particles, and a large number of inflammatory cell infiltrations. The expression levels of inflammatory factors COX-2, TNF-α and IL-6 increased in the tissues(P < 0.05); Compared with the negative control group, the Huashi Runjing Decoction group showed a significant decrease in the area of sodium fluorescein positive staining, a significant decrease in FL score, a significant increase in tear film rupture time and tear secretion, a decrease in serum COX-2, TNF-α and IL-6 levels, a decrease in atrophy of the lacrimal gland tissue vesicles, a more regular shape, a clearer structure, an increase in the number of intracytoplasmic zymogen particles, an increase in inflammatory cell infiltration and a decrease in the area of lacrimal gland tissue. The expression levels of COX-2, TNF-α and IL-6 in lacrimal gland tissues were reduced(P < 0.05). Conclusion Huashi Runjing Decoction could reduce the protein expression of COX-2, TNF-α and IL-6 in the lacrimal gland tissue of castrated male dry eye disease rats, and inhibit the inflammatory response. -
Key words:
- Dry eye disease /
- Huashi Runjing Decoction /
- Inflammatory cytokines /
- COX-2 /
- TNF-α
-
表 1 FL评分细则
Table 1. Scoring rule
阳性染色程度 阳性染色点数 记分 无染色 阴性 0 轻度染色 < 5 1 中度染色 ≥5 2 重度染色 ≥5,伴丝状或块状染色 3 表 2 各组实验大鼠FL评分结果比较[(
$\bar x \pm s$ ),分]Table 2. FL scoring results for each group(
$\bar x \pm s$ ,Points)组别 样本量 造模1周后 用药8周后 假手术组 15 0.60 ± 0.51 0.67 ± 0.49 阴性对照组 15 4.27 ± 0.70△ 4.13 ± 0.83△ 阳性对照组 15 − 2.67 ± 0.72# 化湿润睛汤治疗组 15 − 2.40 ± 0.63# F 202.31 78.48 P < 0.001* < 0.001* *P < 0.05;与假手术组比较,△P < 0.05,与阴性对照组比较,#P < 0.05。 表 3 各组实验大鼠BUT检测结果比较[(
$\bar x \pm s$ ),s]Table 3. BUT test results for each group[(
$\bar x \pm s$ ),s]组别 样本量 造模1周后 用药8周后 假手术组 15 12.27 ± 1.58 12.20 ± 1.52 阴性对照组 15 4.73 ± 0.88△ 5.27 ± 0.88△ 阳性对照组 15 − 7.47 ± 0.92# 化湿润睛汤治疗组 15 − 6.93 ± 0.70# F/渐进F分布 166.34 97.30 P < 0.001* < 0.001* *P < 0.05;与假手术组比较,△P < 0.05,与阴性对照组比较,#P < 0.05。 表 4 各组实验大鼠SIT检测结果比较[(
$\bar x \pm s$ ),mm]Table 4. SIT test results for each group[(
$\bar x \pm s$ ),mm]组别 样本量 造模1周后 用药8周后 假手术组 15 14.00 ± 1.65 14.27 ± 0.88 阴性对照组 15 6.80 ± 1.21△ 7.00 ± 1.13△ 阳性对照组 15 − 11.27 ± 1.44# 化湿润睛汤治疗组 15 − 11.73 ± 1.49# F 99.19 79.93 P < 0.001* < 0.001* *P < 0.05;与假手术组比较,△P < 0.05,与阴性对照组比较,#P < 0.05。 表 5 各组实验大鼠血清COX-2、TNF-α、IL-6 水平变化结果比较[(
$\bar x \pm s$ ),pg/mL]Table 5. Levels of COX-2、TNF-α、IL-6 in serum of each group [(
$\bar x \pm s$ ),pg/mL]组别 样本量 COX-2 TNF-α IL-6 假手术组 15 35.79 ± 7.61 34.09 ± 10.87 35.41 ± 10.32 阴性对照组 15 81.49 ± 8.18△ 74.45 ± 11.35△ 84.27 ± 10.44△ 阳性对照组 15 79.37 ± 7.2 81.31 ± 10.34 82.98 ± 10.81 化湿润睛汤治疗组 15 57.18 ± 7.67#▲ 51.28 ± 7.81#▲ 57.6 ± 7.73#▲ F 161.38 73.77 106.22 P < 0.001* < 0.001* < 0.001* *P < 0.05;与假手术组比较,△P < 0.05;与阴性对照组比较,#P < 0.05;与阳性对照组比较,▲P < 0.05。 表 6 各组实验大鼠泪腺组织中COX-2、TNF-α、IL-6蛋白表达情况(
$\bar x \pm s$ ,n = 3)Table 6. COX2,TNF-α,and IL-6 protein expression in lacrimal gland tissues of experimental rats in each group(
$\bar x \pm s$ ,n = 3)组别 COX-2 TNF-α IL-6 假手术组 2.06 ± 0.05 2.49 ± 0.25 2.36 ± 0.25 阴性对照组 2.90 ± 0.10△ 3.58 ± 0.22△ 3.07 ± 0.18△ 阳性对照组 2.79 ± 0.14 3.19 ± 0.22 2.98 ± 0.29 化湿润睛汤治疗组 1.93 ± 0.05#▲ 2.27 ± 0.18#▲ 2.38 ± 0.15#▲ F 103.84 22.559 12.977 P < 0.001* < 0.001* 0.001* *P < 0.05;与假手术组比较,△P < 0.05;与阴性对照组比较,#P < 0.05;与阳性药物治疗组比较,▲P < 0.05。 -
[1] Wirta D. Update on dry eye disease[J]. Jama,2022,327(23):2355-2356. [2] Lifei Y. Recent developments about the pathogenesis of dry eye disease: Based on immune inflammatory mechanisms [J]. Frontiers in pharmacology, 2021, 12: 732887. 补充期号Lifei Y. Recent developments about the pathogenesis of dry eye disease: Based on immune inflammatory mechanisms[J]. Frontiers in Pharmacology,2021,12:732887. [3] 孙子雯,崔洪玮,孙喜灵,等. 干眼病的病因、发病机制及治疗进展[J]. 山东大学耳鼻喉眼学报,2019(2):159-166. doi: 10.6040/j.issn.1673-3770.0.2018.411 [4] 邢悦,张晓梅. 干眼病因的研究进展[J]. 中国现代医学杂志,2021,31(18):49-54. doi: 10.3969/j.issn.1005-8982.2021.18.010 [5] Borrelli M,Frings A,Geerling G,et al. Gender-specific differences in signs and symptoms of dry eye disease[J]. Curr Eye Res,2021,46(3):294-301. doi: 10.1080/02713683.2020.1801758 [6] Eric B, Papas. The global prevalence of dry eye disease: A bayesian view[J]. Ophthalmic & Physiological Optics: The Journal of the British College of Ophthalmic Opticians (Optometrists),2021,41(6):1254-1266. [7] Mehra D,Galor A. Digital screen use and dry eye: A review[J]. Asia-Pacific Journal of Ophthalmology (Philadelphia,Pa),2020,9(6):491-497. doi: 10.1097/APO.0000000000000328 [8] O'Neil E C,Henderson M,Massaro-Giordano M,et al. Advances in dry eye disease treatment[J]. Current Opinion in Ophthalmology,2019,30(3):166-178. doi: 10.1097/ICU.0000000000000569 [9] 桂炎香,李青松,赵黎,等. 干眼的中医治疗进展[J]. 现代中西医结合杂志,2020,29(9):1018-1021. doi: 10.3969/j.issn.1008-8849.2020.09.026 [10] 董玉,王鹏,张昆. 苏藩主任辨证分型治疗干眼症60例[J]. 云南中医中药杂志,2012,33(5):3-5. doi: 10.3969/j.issn.1007-2349.2012.05.002 [11] 张燕,谢军,徐艺玫,等. 去势大鼠干眼症模型眼表损伤的研究[J]. 生物医学工程研究,2013,32(2):97-100. doi: 10.3969/j.issn.1672-6278.2013.02.008 [12] 李海中,彭清华,王芬,等. 密蒙花总黄酮对去势雄鼠干眼病血清睾酮水平的影响[J]. 国际眼科杂志,2013,13(11):2174-2178. doi: 10.3980/j.issn.1672-5123.2013.11.03 [13] 中华医学会眼科学分会角膜病学组. 干眼临床诊疗专家共识(2013年)[J]. 中华眼科杂志,2013,49(1):73-75. [14] 《中成药治疗优势病种临床应用指南》标准化项目组. 中医药治疗干眼临床应用指南(2021年)[J]. 中国中西医结合杂志,2022,42(9):1040-1046. [15] Ganesalingam K,Ismail S,Sherwin T,et al. Molecular evidence for the role of inflammation in dry eye disease[J]. Clinical & Experimental Optometry,2019,102(5):446-454. [16] Wang L,Deng Y. The applications of androgen in the treatment of dry eye disease: A systematic review of clinical studies[J]. Endocrine journal,2020,67(9):893-902. doi: 10.1507/endocrj.EJ20-0178 [17] ruong S,Cole N,Stapleton F,et al. Sex hormones and the dry eye[J]. Clinical & Experimental Optometry,2014,97(4):324-336. [18] Song X,Zhao P,Wang G,Zhao X,et al. The effects of estrogen and androgen on tear secretion and matrix metalloproteinase-2 expression in lacrimal glands of ovariectomized rats[J]. Investigative Ophthalmology & Visual Science,2014,55(2):745-751. [19] 谢翔,应炜阳,金胜威. COX-2在炎症消退中的研究进展[J]. 生命的化学,2016,36(4):461-464. doi: 10.13488/j.smhx.20160406 [20] Kulkarni P S,Srinivasan B D. Cyclooxygenase and lipoxygenase pathways in anterior uvea and conjunctiva[J]. Progress in Clinical and Biological Research,1989,312:39-52. [21] Shim J,Park C,Lee H S,et al. Change in prostaglandin expression levels and synthesizing activities in dry eye disease[J]. Ophthalmology,2012,119(11):2211-2219. doi: 10.1016/j.ophtha.2012.05.038 [22] Ji Y W,Seo Y,Choi W,et al. Dry eye-induced CCR7+CD11b+ cell lymph node homing is induced by COX-2 activities[J]. Investigative Ophthalmology & Visual Science,2014,55(10):6829-6838. [23] Ueta M. Genetic predisposition to stevens-johnson syndrome with severe ocular surface complications[J]. Cornea,2015,34(Suppl 11):S158-165. [24] 马玉,李研,骆亚莉,等. 肿瘤坏死因子-α在慢性炎症与肿瘤发生中的作用[J]. 中国临床药理学杂志,2022,38(12):1419-1423. doi: 10.13699/j.cnki.1001-6821.2022.12.027 -





 下载:
下载: