Efficacy and Safety of Sofosbuvir/Velpatasvir with or without Ribavirin in Cirrhotic Patients with Genotype 3 Chronic Hepatitis C Infection
-
摘要:
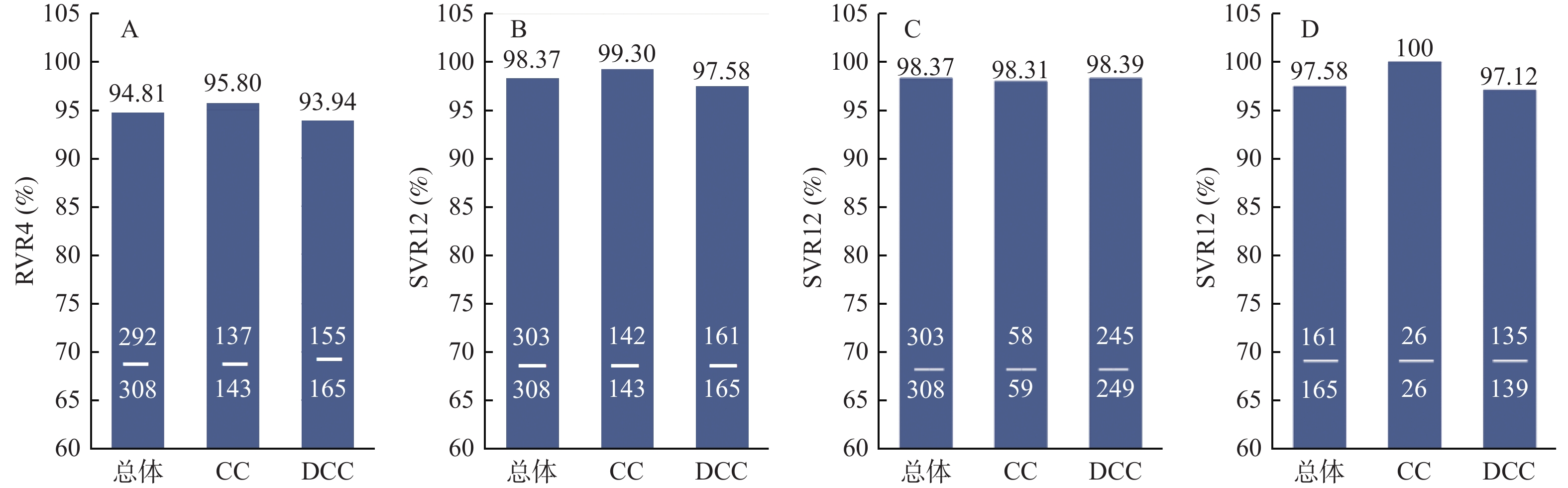
目的 探讨索磷布韦/维帕他韦±利巴韦林(sofosbuvir/velpatasvir ± ribavirin,SOF/VEL±RBV)治疗基因3型丙型肝炎肝硬化患者的疗效和安全性。 方法 回顾性纳入2018年6月至2023年2月就诊于昆明市第三人民医院诊断为基因3型(GT3)肝硬化患者,使用SOF/VEL+RBV治疗12周,如有RBV禁忌或者RBV不耐受,则采用SOF/VEL治疗12或24周。分析患者在治疗前、治疗4周、12周及停药后12周的病毒学指标、肝肾功能指标和不良反应等。 结果 最终纳入319例GT3肝硬化患者,其中SOF/VEL+RBV组308例,SOF/VEL组11例。停药12周后,SOF/VEL+RBV组持续病毒学应答(SVR12)率达98.37%(303/308),与基线相比,APRI评分和FIB-4指数水平均下降(P < 0.05),总胆红素、天冬氨酸转氨酶、丙氨酸转氨酶水平均下降,差异均有统计学意义(均P < 0.05)。SOF/VEL组SVR12率为72.73%(8/11)。SOF/VEL+RBV组的不良反应为轻度溶血性贫血(15.26%)、乏力(8.12%)和皮疹(8.77%),SOF/VEL组为1例乏力(9.09%)。 结论 索磷布韦/维帕他韦联合利巴韦林方案在基因3型丙肝感染的代偿期肝硬化和失代偿期肝硬化患者中都能获得较高SVR12率(98.37%),生化学指标和肝纤维化程度均较治疗前有所改善且安全性好。 Abstract:Objective To investigate the efficacy and safety of Sofosbuvir/Velpatasvir ± ribavirin(SOF/VEL±RBV) in the treatment of genotype 3 chronic hepatitis C cirrhotic patients. Methods Patients diagnosed with genotype 3(GT3) HCV infection and treated at the Third People's Hospital of Kunming City from June 2018 to February 2023 were retrospectively included. All patients had liver cirrhosis and were treated with SOF/VEL+RBV for 12 weeks, SOF/VEL therapy for 12 or 24 weeks if favorable ribavirin contrainminated or ribavirin intolerant. The virologic indexes, liver and kidney function indexes and adverse reactions of the patients were analyzed before treatment, 4 weeks, 12 weeks and 12 weeks after drug withdrawal. Results A total of 319 patients were included, including 308 cases in SOF/VEL+RBV group and 11 cases in SOF/VEL group. After 12 weeks off-treatment, the sustained virological response(SVR12) rate in SOF/VEL+ RBV group was 98.37%(303/308), and the levels of APRI score and FIB-4 index were decreased compared with baseline(P < 0.05). The levels of total bilirubin, aspartate aminotransferase and alanine aminotransferase were all decreased, and the differences were statistically significant(all P < 0.05). The SVR12 rate of SOF/VEL group was 72.73%(8/11). The adverse reactions were mild hemolytic anemia(15.26%), fatigue(8.12%) and rash(8.77%) in SOF/VEL+RBV group, and fatigue(9.09%) in 1 case in SOF/VEL group. Conclusion SOF/VEL+RBV could achieve higher SVR12 and well tolerated for GT3 HCV-infected patients either with compensated cirrhosis or decompensated cirrhosis patients. Liver function, renal function and liver fibrosis could also be improved after treatment. -
Key words:
- Sofosbuvir/Velpatasvir /
- Chronic hepatitis C /
- Genotype 3 /
- Liver cirrhosis /
- Virological response
-
表 1 SOF/VEL+RBV组患者的基线临床特征[(
$\bar x \pm s $ )/n (%)/M(P25,P75)]Table 1. Baseline clinical features of patients in the SOF/VEL+RBV group [(
$\bar x \pm s $ )/n (%)/M(P25,P75)]项目 总数(n =308) CC (n = 143) DCC (n = 165) t/χ2/H P 年龄(岁) 50.51 ± 7.83 50.00 ± 7.54 50.89 ± 8.03 0.942 0.347 性别(男/女) 228/80 112/31 116/49 2.562 0.109 HCV RNA (lgIU/mL) 5.78 ± 1.09 5.89 ± 1.08 5.70 ± 1.09 1.259 0.210 RBC (× 1012/L) 4.12 ± 1.05 4.66 ± 0.95 3.77 ± 0.96 6.678 < 0.001* HGB (g/L) 135.09 ± 32.69 151.33 ± 28.63 124.13 ± 30.73 6.479 < 0.001* TBIL (µmol/L) 19.65(13.83,31.13) 17.30(13.20,20.90) 22.70(14.55,41.60) 16.733 < 0.001* AST (U/L) 68.61 ± 38.49 64.47 ± 38.05 70.80 ± 38.73 0.973 0.033* ALT (U/L) 65.15 ± 40.02 78.38 ± 45.34 56.51 ± 33.59 3.962 < 0.001* GGT (U/L) 84.87 ± 50.31 75.50 ± 38.96 90.96 ± 55.83 2.125 0.035* ALP (g/L) 122.82 ± 41.74 108.83 ± 33.79 132.28 ± 44.03 3.901 < 0.001* ALB (g/L) 41.93 ± 16.58 40.27 ± 7.85 43.16 ± 20.76 1.360 0.176 CHE (U/L) 4183.11 ± 1866.58 5584.79 ± 1477.86 3373.53 ± 1566.78 9.537 < 0.001* CREA (μmol/L) 72.44 ± 28.61 67.38 ± 16.96 75.84 ± 33.93 2.357 0.190 UREA (mmol/L) 387.71 ± 133.69 366.65 ± 100.12 403.15 ± 152.29 2.047 0.042* 基因型 3a 59(19.16) 35(24.48) 24(14.55) 4.878 0.027* 3b 249(80.84) 108(75.52) 141(85.45) 合并疾病 HIV 43(13.96) 20(13.99) 23(13.94) 0.002 0.991 高血压 35(11.36) 13(9.09) 22(13.33) 1.847 0.174 糖尿病 56(18.18) 18(12.59) 38(23.03) 5.616 0.018* RBC:红细胞 HGB:血红蛋白 TBIL:总胆红素 AST:门冬氨酸氨基转移酶 ALT:丙氨酸氨基转移酶 GGT:γ-谷氨酰基转移酶 ALP:碱性磷酸酶 ALB:白蛋白 CHE:胆碱酯酶 CREA:肌酐 UREA:尿素; *P < 0.05。 表 2 基因3型慢性丙型肝炎肝硬化患者的基线临床特征[n /M(P25,P75)]
Table 2. Baseline clinical characteristics of patients with genotype 3 chronic hepatitis C cirrhosis [n /M(P25,P75)]
项目 SOF/VEL+RBV组(n = 308) SOF/VEL组(n = 11) t / H P 年龄(岁) 50.00(46.00,56.00) 50.00(45.00,54.00) 0.312 0.577 性别(男/女) 228/80 8/3 0.001 1.000 肝硬化(失代偿/代偿) 165/143 7/4 0.433 0.511 HCV RNA (lgIU/mL) 6.04(5.32,6.50) 6.06(4.44,6.57) 0.074 0.785 TBIL (µmol/L) 19.65(13.83,31.13) 19.50(11.50,31.20) 0.100 0.751 AST (U/L) 58.00(40.00,87.00) 36.00(27.75,60.75) 3.777 0.052 ALT (U/L) 57.00(37.00,83.00) 53.00(32.00,67.00) 0.505 0.477 表 3 SOF/VEL+RBV组治疗前后APRI和FIB-4评分的变化[
$ \bar x \pm s $ ]Table 3. Changes of APRI and FIB-4 scores before and after treatment in SOF/VEL+RBV group [
$ \bar x \pm s $ ]时间 APRI评分 FIB-4指数 CC DCC t P CC DCC t P 基线 2.13 ± 1.29 2.66 ± 1.94 2.011 0.046* 4.73 ± 3.10 6.90 ± 4.33 3.202 0.002* 治疗结束后12周 1.06 ± 0.43 1.74 ± 1.34 3.475 0.001* 3.10 ± 2.15 5.33 ± 3.38 4.296 0.006* t 5.581 4.081 3.526 2.773 P < 0.001* < 0.001* 0.001* 0.006* *P < 0.05。 表 4 SOF/VEL组治疗前后APRI和FIB-4评分的变化 [M(P25,P75) ]
Table 4. Changes of APRI and FIB-4 scores before and after treatment in SOF/VEL group [ M(P25,P75)]
时间 APRI评分 FIB-4指数 CC DCC H P CC DCC H P 基线 1.90(1.70,2.52) 2.58(1.64,2.77) 0.378 0.539 4.05(2.74,6.76) 2.16(1.32,2.25) 6.000 0.014* 治疗结束后12周 1.50(0.87,1.70) 1.83(0.98,2.58) 0.540 0.462 3.27(2.57,5.75) 2.28(1.63,3.08) 2.940 0.086 H 3.000 1.320 0.333 0.884 P 0.083 0.251 0.564 0.347 *P < 0.05。 表 5 SOF/VEL+RBV组治疗前后肝功能、肾功能和血常规的变化[(
$ \bar x \pm s $ )/M(P25,P75)]Table 5. Changes of liver function,renal function and blood routine in SOF/VEL+RBV group before and after treatment [(
$ \bar x \pm s $ )/M(P25,P75)]项目 基线 治疗后 t/H P TBIL (µmol/L) 总体 19.65(13.83,31.13) 16.70(11.55,24.88) 8.091 0.004* CC 17.30(13.20,20.90) 15.80(10.50,21.25) 1.373 0.241 DCC 22.70(14.55,41.60) 18.50(12.45,27.55) 8.265 0.004*
AST(U/L)总体 68.61 ± 38.49 36.49 ± 21.63 8.999 < 0.001* CC 64.47 ± 38.05 29.70 ± 17.24 6.060 < 0.001* DCC 70.80 ± 38.73 40.98 ± 22.90 6.827 < 0.001* ALT(U/L) 总体 65.15 ± 40.02 26.64 ± 19.06 12.439 < 0.001* CC 78.38 ± 45.34 25.59 ± 17.98 9.741 < 0.001* DCC 56.50 ± 33.59 27.33 ± 19.78 8.336 < 0.001* GGT(U/L) 总体 83.12 ± 45.96 50.80 ± 58.14 5.685 < 0.001* CC 75.50 ± 38.96 40.09 ± 23.75 6.354 < 0.001* DCC 88.07 ± 49.54 51.95 ± 34.70 6.060 < 0.001* CHE(U/L) 总体 4205.42 ± 1847.12 4548.29 ± 1879.93 1.755 0.080 CC 5584.79 ± 1477.86 5726.43 ± 1615.37 0.530 0.597 DCC 3401.78 ± 1543.66 3861.89 ± 1676.14 2.165 0.031* CREA (μmol/L) 总体 72.20 ± 28.80 71.22 ± 31.25 0.325 0.745 CC 67.37 ± 16.96 68.06 ± 17.13 0.261 0.794 DCC 75.74 ± 34.05 73.47 ± 38.18 0.482 0.630 HGB(g/L) 总体 135.09 ± 32.69 126.05 ± 32.86 2.832 0.005* CC 151.33 ± 28.63 137.73 ± 29.52 3.049 0.003* DCC 124.13 ± 30.73 118.17 ± 32.77 1.489 0.138
RBC(1012/L)总体 4.12 ± 1.05 3.91 ± 1.07 2.105 0.036* CC 4.66 ± 0.95 4.22 ± 1.01 2.949 0.004* DCC 3.77 ± 0.96 3.71 ± 1.06 0.512 0.609 *P < 0.05。 表 6 SOF/VEL组治疗前后肝功能、肾功能和血常规的变化[ M(P25,P75)]
Table 6. Changes of liver function,renal function and blood routine in SOF/VEL group before and after treatment [M(P25,P75)]
项目 基线 治疗后 H P TBIL(µmol/L) 总体 19.50(11.50,31.20) 21.10(12.40,37.40) 0.053 0.818 CC 17.55(11.95,36.95) 19.10(11.40,33.18) 0.083 0.773 DCC 19.50(9.90,31.20) 23.10(12.40,38.00) 0.200 0.655 AST(U/L) 总体 36.00(27.75,60.75) 27.50(26.25,31.00) 5.389 0.020* CC 49.50(31.50,70.50) 28.50(24.75,33.00) 3.000 0.083 DCC 29.00(27.00,60.75) 27.50(25.50,83.25) 2.619 0.106 ALT(U/L) 总体 53.00(32.00,67.00) 20.00(14.00,24.00) 8.168 0.004* CC 49.50(14.75,89.50) 19.00(15.75,21.50) 1.333 0.248 DCC 53.00(37.00,66.00) 22.00(12.00,27.00) 6.876 0.009* GGT(U/L) 总体 126.00(51.00,287.00) 54.00(43.00,71.00) 3.382 0.066 CC 95.00(29.00,286.25) 32.50(17.10,145.25) 0.750 0.386 DCC 133.00(51.00,287.00) 56.00(46.00,71.00) 2.551 0.110 CHE(U/L) 总体 3349.00(2443.00,5311.00) 3796.00(2890.00,6111.00) 0.674 0.412 CC 3723.00(2669.50,6825.50) 4388.50(3292.00,7201.00) 0.333 0.564 DCC 2729.00(2306.00,5311.00) 3791.00(2245.00,6111.00) 0.494 0.482 CREA (μmol/L) 总体 85.00(65.00,291.00) 80.00(58.00,140.00) 0.570 0.450 CC 304.50(63.50,613.75) 315.50(63.25,969.75) 0.083 0.773 DCC 85.00(78.00,284.00) 80.00(57.00,95.00) 1.800 0.180 HGB(g/L) 总体 119.50(98.25,141.75) 122.00(102.50,138.25) 0.006 0.940 CC 113.00(84.75,151.00) 121.50(77.50,134.75) 0.001 1.000 DCC 116.50(97.25,141.75) 124.00(101.25,152.75) 0.001 1.000 RBC(1012/L) 总体 3.78(2.95,4.39) 3.80(2.92,4.21) 0.013 0.910 CC 3.66(2.66,4.64) 3.80(2.36,4.04) 0.021 0.885 DCC 3.87(2.78,4.39) 3.67(2.87,4.87) 0.161 0.688 *P < 0.05。 表 7 SOF/VEL+RBV组患者的不良反应 [n (%)]
Table 7. Adverse reactions in SOF/VEL+RBV patients [n (%)]
不良反应 总体(n = 308) CC(n = 143) DCC(n = 165) 全部不良反应 140(45.45) 59(41.26) 81(49.09) 溶血性贫血 47(15.26) 22(15.38) 25(15.15) 皮肤瘙痒 19(6.17) 6(4.20) 13(7.88) 皮疹 27(8.77) 11(7.69) 16(9.70) 眩晕 22(7.14) 9(6.29) 13(7.88) 乏力 25(8.12) 11(7.69) 14(8.48) -
[1] Gao Y,Yang J,Sun F,et al. Prevalence of Anti-HCV antibody among the general population in China's mainland between 1991 and 2015: A systematic review and meta-analysis[J]. Open Forum Infect Dis,2019,6(3):1-7.Gao Y,Yang J,Sun F,et al. Prevalence of Anti-HCV antibody among the general population in China's mainland between 1991 and 2015: A systematic review and meta-analysis[J]. Open Forum Infect Dis,2019,6(3):1-7. [2] Mei X, Lu H. Prevalence, diagnosis, and treatment of hepatitis C in China's mainland[J/OL]. Glob Health Med, 2021, 3(5): 270-275.Mei X, Lu H. Prevalence, diagnosis, and treatment of hepatitis C in China's mainland[J/OL]. Glob Health Med, 2021, 3(5): 270-275. [3] Qin Q,Smith M K,Wang L,et al. Hepatitis C virus infection in China: An emerging public health issue[J]. J Viral Hepat,2015,22(3):238-244. doi: 10.1111/jvh.12295 [4] Yuanyuan J,Xiu Z,Wei Y,et al. The distribution of hepatitis C viral genotypes shifted among chronic hepatitis C patients in Yunnan,China,between 2008–2018[J]. Frontiers in Cellular and Infection Microbiology,2023,11(7):92-93. [5] Tayyab G U N,Rasool S,Nasir B,et al. Hepatocellular carcinoma occurs frequently and early after treatment in HCV genotype 3 infected persons treated with DAA regimens[J]. BMC Gastroenterol,2020,20(1):93. doi: 10.1186/s12876-020-01249-4 [6] 陈红松,窦晓光,段钟平,等. 丙型肝炎防治指南(2015年更新版)[J]. 临床肝胆病杂志,2015,31(12):1961-1979. doi: 10.3969/j.issn.1001-5256.2015.12.003 [7] 中华医学会肝病学分会,中华医学会感染病学分会. 丙型肝炎防治指南(2022年版)[J]. 中华传染病杂志,2023,41(1):29-46. doi: 10.3760/cma.j.cn311365-20230217-00045 [8] Xia X,Luo J,Bai J,et al. Epidemiology of hepatitis C virus infection among injection drug users in China: Systematic review and meta-analysis[J]. Public Health,2008,122(10):990-1003. doi: 10.1016/j.puhe.2008.01.014 [9] Lauer G M,Walker B D. Hepatitis C virus infection[J]. N Engl J Med,2001,345(1):41-52. doi: 10.1056/NEJM200107053450107 [10] 关富,王胜炳,张鸣青. 《2022年美国肝病学会实践指南:肝硬化失代偿期的症状管理和姑息性治疗》摘译[J]. 临床肝胆病杂志,2022,38(4):784-787. [11] 叶晓婷,徐霜,张盛果,等. 索磷布韦/维帕他韦治疗基因3型和6型慢性丙型肝炎患者的疗效和安全性[J]. 温州医科大学学报,2023,53(8):662-666. doi: 10.3969/j.issn.2095-9400.2023.08.009 [12] 中华医学会肝病学分会,中华医学会感染病学分会. 丙型肝炎防治指南(2019年版)[J]. 中华肝脏病杂志,2019,27(12):962-979. doi: 10.3760/cma.j.issn.1007-3418.2019.12.008 [13] Fabrizi F,Cerutti R,Dixit V,et al. Sofosbuvir-based regimens for HCV in stage 4-stage 5 chronic kidney disease. A systematic review with meta-analysis[J]. Nefrologia (Engl Ed),2021,41(5):578-589. [14] 江啸,姜晓娟,苏锐良,等. 基于索非布韦的方案联合利巴韦林治疗肝移植后HCV基因1型肝炎受者的Meta分析[J]. 器官移植,2019,10(5):570-577. doi: 10.3969/j.issn.1674-7445.2019.05.017 [15] 冯巩,宋娟娟,叶峰,等. 肝硬化再代偿:现状与挑战[J]. 临床肝胆病杂志,2023,39(10):2464-2469. [16] 白金锡,白贞子,郑艳. 36例慢性丙型肝炎抗病毒副作用及对策[J]. 中国实验诊断学,2013,17(2):346-347. [17] 赵智蓉,李海雯,李晓非,等. 索磷布韦维帕他韦联合利巴韦林治疗基因3型慢性丙肝患者的疗效与安全性[J]. 昆明医科大学学报,2021,42(3):98-103. [18] 魏来, 谢青, 黄燕, 等. 索磷布韦/维帕他韦用于基因1-6型HCV中国患者时的安全性和疗效: 来自一项3期临床试验的结果[C]. 第十届全国疑难及重症肝病大会, 苏州, 2019. [19] Esteban R,Pineda J A,Calleja J L,et al. Efficacy of sofosbuvir and velpatasvir,with and without ribavirin,in patients with hepatitis C virus genotype 3 infection and cirrhosis[J]. Gastroenterology,2018,155(4):1120-1127.e4. doi: 10.1053/j.gastro.2018.06.042 -






 下载:
下载: