Role of Mertk-mediated NF-κb Pathway in Inflammatory Response of Schwann Cells
-
摘要:
目的 探究Mertk表达水平对大鼠雪旺细胞NF-κb通路的调控作用及其可能的机制。 方法 体外培养大鼠雪旺细胞,免疫印迹法检测Mertk在高糖环境雪旺细胞中表达情况。免疫共沉淀法检测内源性Mertk与Ikbkb相互作用。免疫印迹法检测沉默Mertk后雪旺细胞内Ikbkb、P65、肿瘤坏死因子-α表达水平。免疫荧光法检测沉默Mertk后Mertk、Ikbkb、P65蛋白表达。 结果 Mertk在雪旺细胞中存在表达,且表达量随葡萄糖浓度增加而提高;免疫共沉淀实验表明Mertk与Ikbkb在大鼠雪旺细胞内相互作用;免疫印迹实验显示,将雪旺细胞内Mertk敲减表达后,与对照组相比,Mertk表达水平降低(P < 0.05),Ikbkb、P65、TNF-α明显升高(P < 0.05);免疫荧光实验显示,沉默后的雪旺细胞内Mertk荧光减弱,Ikbkb、P65荧光增强。 结论 Mertk表达降低后,可以通过与Ikbkb进行相互作用,介导调控雪旺细胞NF-κb通路,上调P65和炎症因子TNF-α的表达。 Abstract:Objective To explore the regulatory effect of Mertk expression level on NF-κb pathway in rat Schwann cells and its possible mechanism. Methods Rat Schwann cells were cultured in vitro, and the expression of Mertk in Schwann cells exposed to high glucose was detected by Western blot. Co-immunoprecipitation was used to detect the interaction between endogenous Mertk and Ikbkb. Western blot was used to detect the expression levels of Ikbkb, P65 and tumor necrosis factor-α in Schwann cells after Mertk silencing. The protein expressions of Mertk, Ikbkb and P65 after silencing Mertk were detected by immunofluorescence. Results Mertk was expressed in Schwann cells, and the expression level increased with the increase of glucose concentration. Co-immunoprecipitation assay showed that Mertk interacted with Ikbkb in rat Schwann cells. Compared with the control group, the expression level of Mertk was significantly decreased(P < 0.05), while Ikbkb, P65 and TNF-α were significantly increased(P < 0.05) after knock down expression of Mertk in Schwann cells. Immunofluorescence experiments showed that the fluorescence of Mertk was decreased, and the fluorescence of Ikbkb and P65 was increased in the silenced Schwann cells. Conclusion After the expression of Mertk is decreased, it can mediate the regulation of NF-κb pathway in Schwann cells through interaction with Ikbkb, and up-regulate the expression of P65 and inflammatory factor TNF-α. -
Key words:
- Mertk /
- Ikbkb /
- Nuclear factor kappa-b /
- Schwann cells /
- Diabetes peripheral neuropathy
-
表 1 Mertk小干扰核苷酸序列
Table 1. Mertk-RNA oligo sequences
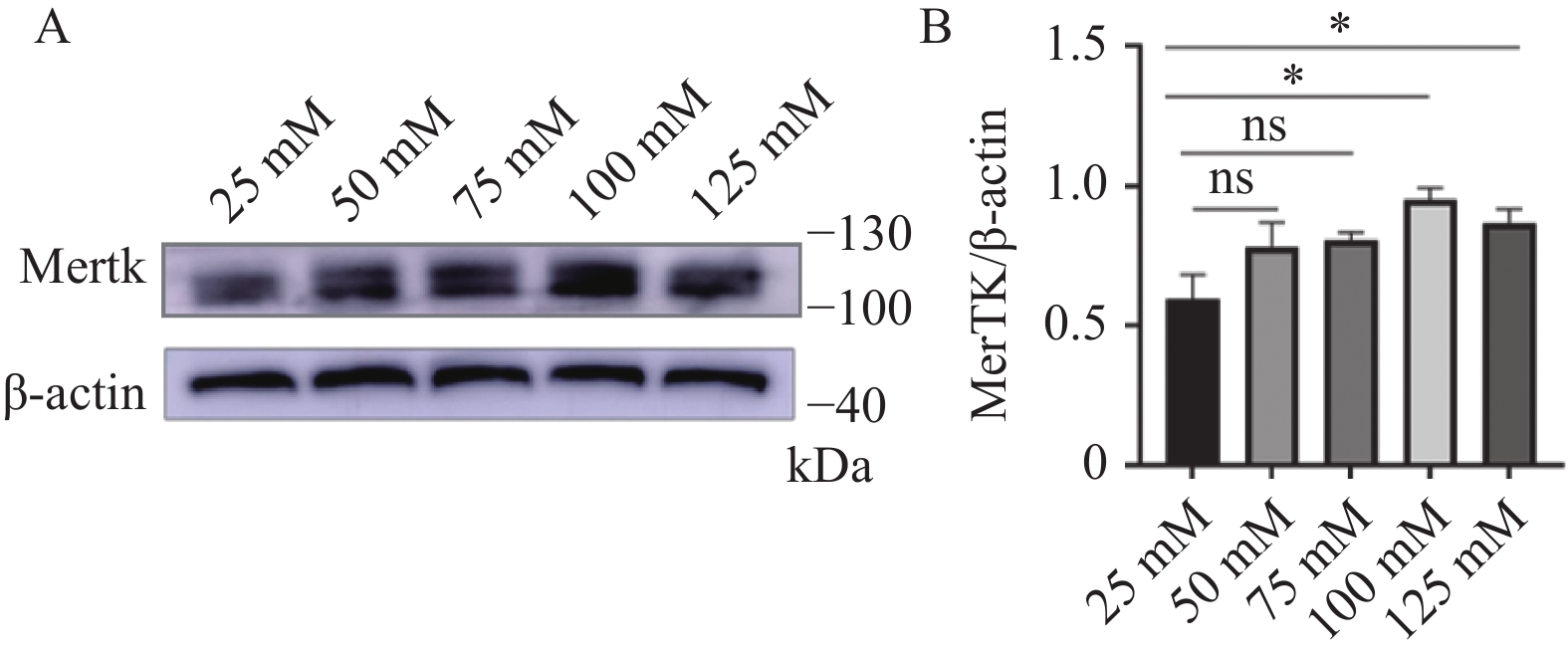
寡核苷酸 正义序列(5′-3′) 反义序列(5′-3′) Mertk-Rat-1 GGGUCGUACAUCUGUAAGATT UCUUACAGAUGUACGACCCTT Mertk-Rat-2 CACCCACUGAAGUCCAUAUTT AUAUGGACUUCAGUGGGUGTT Mertk-Rat-3 GAGGAAAGAUACAUCUUAATT UUAAGAUGUAUCUUUCCUCTT 表 2 不同葡萄糖水平RSC96内Mertk蛋白表达
Table 2. Mertk protein expression within RSC96 at different glucose levels
灰度值
比值组别 F P 25 mmol/L 50 mmol/L 75 mmol/L 100 mmol/L 125 mmol/L Mertk/β-actin 0.60 ± 0.20 0.78 ± 0.19 0.81 ± 0.06 0.95 ± 0.09* 0.87 ± 0.11* 4.452 0.0098* 与对照组(25 mmol/L组)比较,*P < 0.05。 表 3 沉默Mertk后Ikbkb、P65、TNF-α蛋白表达量
Table 3. Ikbkb,P65,and TNF-α protein expression after silencing Mertk
组别 Mertk
/β-actinIkbkb
/β-actinP65
/β-actinTNF-α
/β-actinCON 1.13 ± 0.22 0.38 ± 0.11 0.60 ± 0.12 0.60 ± 0.09 NC 1.28 ± 022 0.44 ± 0.22 0.57 ± 0.16 0.62 ± 0.19 siRNA1 0.68 ± 0.08* 1.10 ± 0.18* 1.22 ± 0.18* 1.05 ± 0.25* siRNA2 0.60 ± 0.24* 1.12 ± 0.02* 1.02 ± 0.09* 0.85 ± 0.04* siRNA3 0.60 ± 0.12* 1.18 ± 0.28* 0.95 ± 0.11* 1.19 ± 0.09* F 8.927 13.76 12.62 8.646 P 0.0025* 0.0004* 0.0006* 0.0028* 与CON组比较,*P < 0.05。 -
[1] Wang W,Ji Q,Ran X,et al. Prevalence and risk factors of diabetic peripheral neuropathy: A population-based cross-sectional study in China[J]. Diabetes Metab Res Rev,2023,39(8):e3702. doi: 10.1002/dmrr.3702 [2] Harty B L,Coelho F,Pease-Raissi S E,et al. Myelinating Schwann cells ensheath multiple axons in the absence of E3 ligase component Fbxw7[J]. Nature Communications,2019,10(1):2976. doi: 10.1038/s41467-019-10881-y [3] Wang L,Chopp M,Szalad A,et al. Exosomes derived from Schwann cells ameliorate peripheral neuropathy in type 2 diabetic mice[J]. Diabetes,2020,69(4):749-759. doi: 10.2337/db19-0432 [4] Hinder L M,Murdock B J,Park M,et al. Transcriptional networks of progressive diabetic peripheral neuropathy in the db/db mouse model of type 2 diabetes: An inflammatory story[J]. Exp Neurol,2018,305:33-43. doi: 10.1016/j.expneurol.2018.03.011 [5] Suzuki T,Sekido H,Kato N,et al. Neurotrophin-3-induced production of nerve growth factor is suppressed in Schwann cells exposed to high glucose: Involvement of the polyol pathway[J]. Journal of Neurochemistry,2004,91(6):1430-1438. doi: 10.1111/j.1471-4159.2004.02824.x [6] Huang Y,Lemke G. Early death in a mouse model of Alzheimer's disease exacerbated by microglial loss of TAM receptor signaling[J]. Proceedings of the National Academy of Sciences of the United States of America,2022,119(41):e2204306119. [7] Pilli VS,Datta A,Dorsey A,et al. Modulation of protein S and growth arrest specific 6 protein signaling inhibits pancreatic cancer cell survival and proliferation[J]. Oncology Reports,2020,44(4):1322-1332. [8] Burstyn-Cohen T,Hochberg A. TAM signaling in the nervous system[J]. Brain Plasticity,2021,7(1):33-46. doi: 10.3233/BPL-210125 [9] Aehnlich P,Powell RM,Peeters MJW,et al. TAM receptor inhibition–implications for cancer and the immune system[J]. Cancers,2021,13(6):1195. doi: 10.3390/cancers13061195 [10] Lee Y J,Han J Y,Byun J,et al. Inhibiting Mer receptor tyrosine kinase suppresses STAT1,SOCS1/3,and NF-κB activation and enhances inflammatory responses in lipopolysaccharide-induced acute lung injury[J]. Journal of Leukocyte Biology,2012,91(6):921-932. doi: 10.1189/jlb.0611289 [11] Lan D,Jiang H Y,Su X,et al. Transcriptome-wide association study identifies genetically dysregulated genes in diabetic neuropathy[J]. Combinatorial Chemistry & High Throughput Screening,2021,24(2):319-325. [12] Chandrasekaran K,Anjaneyulu M,Choi J,et al. Role of mitochondria in diabetic peripheral neuropathy: Influencing the NAD+-dependent SIRT1-PGC-1α-TFAM pathway[J]. International Review of Neurobiology,2019,145:177-209. [13] Eid S A,El Massry M,Hichor M,et al. Targeting the NADPH oxidase-4 and liver X receptor pathway preserves Schwann cell integrity in diabetic mice[J]. Diabetes,2020,69(3):448-464. doi: 10.2337/db19-0517 [14] Li J,Guan R,Pan L. Mechanism of Schwann cells in diabetic peripheral neuropathy: A review[J]. Medicine (Baltimore),2023,102(1):e32653. [15] Capece D,Verzella D,Flati I,et al. NF-κB: Blending metabolism,immunity,and inflammation[J]. Trends in Immunology,2022,43(9):757-775. doi: 10.1016/j.it.2022.07.004 [16] Antonia R J,Hagan R S,Baldwin A S,et al. Expanding the view of IKK: New substrates and new biology[J]. Trends Cell Biol,2021,31(3):166-178. doi: 10.1016/j.tcb.2020.12.003 [17] 公丕昊,马龙飞,孟纯阳. NF-κB在神经性病理疼痛中的作用研究进展[J]. 中国医师杂志,2019,21(9):1438-1441. -






 下载:
下载: