The Investigation and Analysis of the Situation of COVID-19 Vaccination and Vaccination Willingness in HIV/AIDS Population in Yunnan Province
-
摘要:
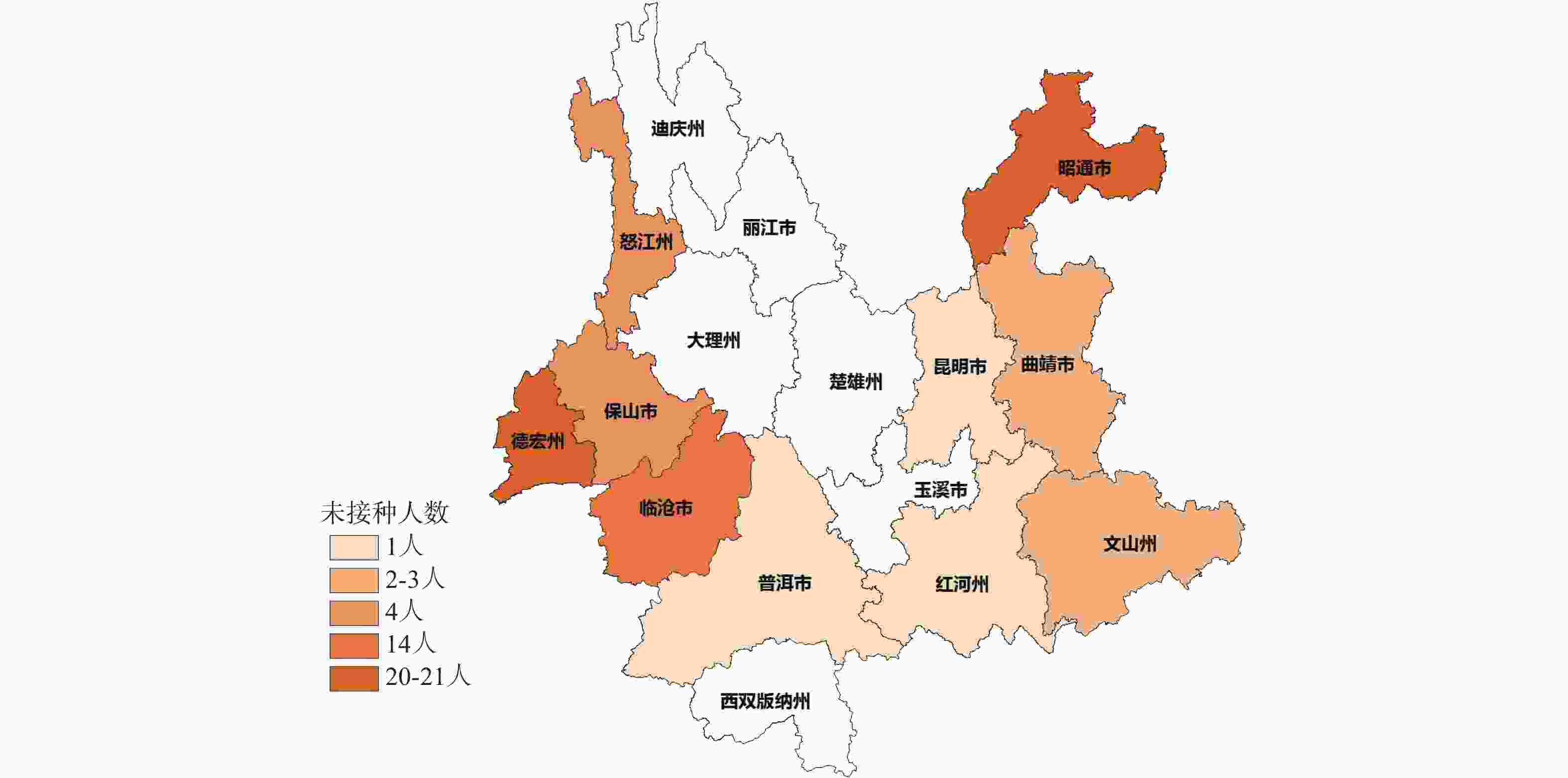
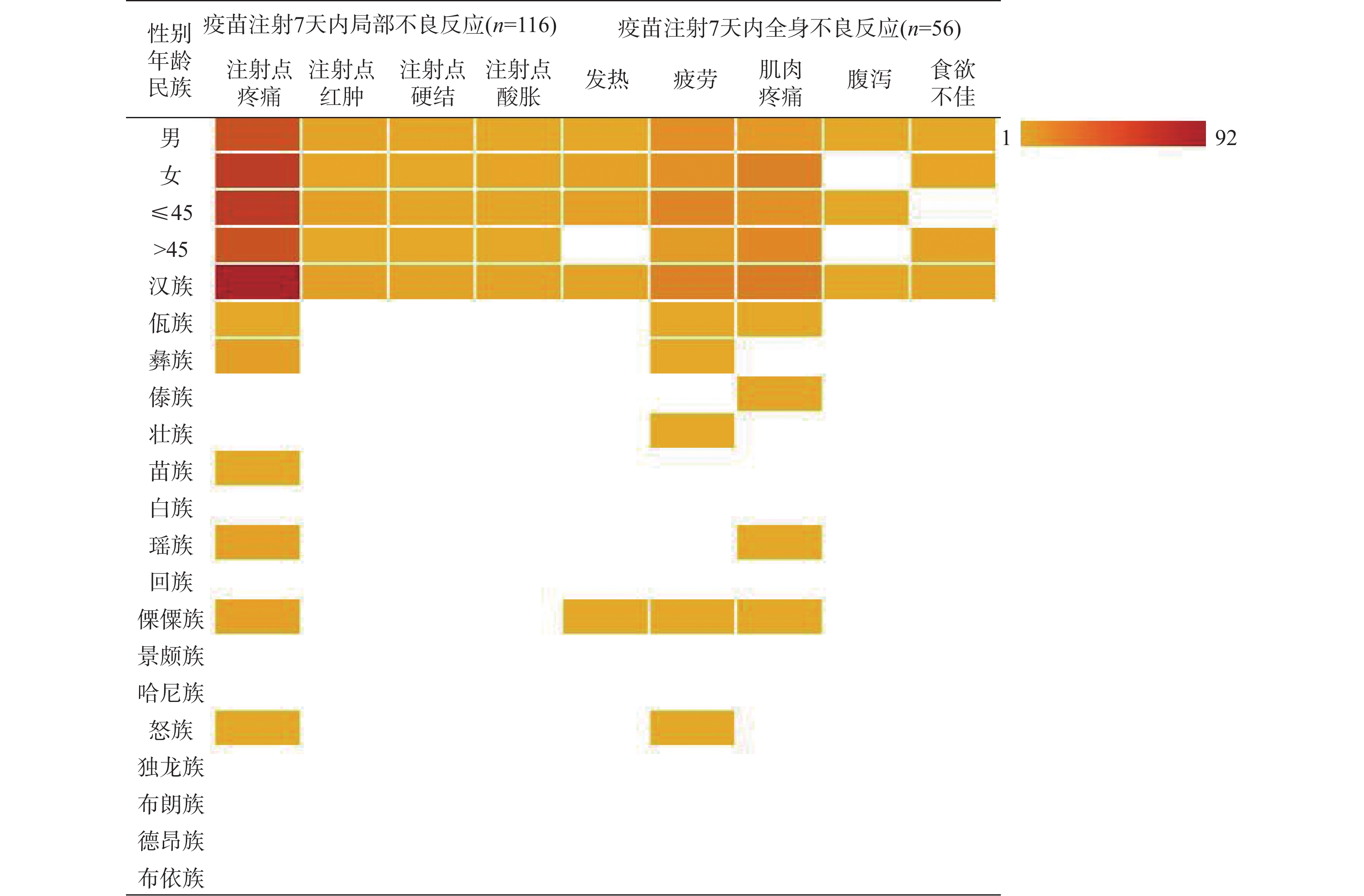
目的 调查分析云南地区HIV/AIDS人群新型冠状病毒疫苗接种情况、接种意愿及影响该人群接种意愿的影响因素。 方法 2021年10月至2022年6月期间采用自制问卷形式对云南省昆明市、曲靖市、玉溪市、昭通市、普洱市、保山市、临沧市、红河州、文山州、西双版纳州、大理州、德宏州、怒江州13个州市医院随访的2180名HIV/AIDS患者进行现场调查。问卷考评通过,内容包括年龄、性别、学历、民族、文化程度、疫苗接种情况、接种后7 d内不良反应,对新冠疫苗安全、效用认知程度,接种意愿等方面的内容。 结果 调查对象中2109名完成了3针新冠疫苗接种,占比96.74%,有71名未接种,占比3.26%。接种7 d内局部出现不良反应116名,占比5.50%,全身出现不良反应56名,占比2.66%。不同性别、年龄段及汉族中注射点疼痛、疲劳和肌肉疼痛是占比最高的不良症状,少数民族不良反应占比很低,不同性别及年龄不良反应占比差异无统计学意义(P > 0.05)。HIV/AIDS人群不愿意接种疫苗的主要原因是(医师建议)HIV/AIDS患者不能接种疫苗(67.61%)和接种后可能有严重不良反应(19.72%),应用Logistic回归分析影响疫苗接种的因素发现是否担心感染新冠病毒(OR = 0.121,95%CI = 0.083~0.640,P < 0.001)、对新冠疫苗了解程度(OR = 28.932,95%CI = 15.469~54.115,P < 0.001)、接种疫苗是否安全(OR = 13.953,95%CI =4.819~40.404,P < 0.001)及是否相信疫苗的预防作用(OR = 14.017,95%CI = 4.752~41.348,P < 0.001)是影响疫苗接种的显著因素, 13个州市中德宏州(20人)、昭通市(21人)、临沧市(14人)未接种人数最多。 结论 HIV/AIDS人群大规模接种疫苗后不良反应比率低,症状轻,医师正确、科学的建议和指导以及充分了解疾病的危害性,疫苗的安全性、预防性、有效性是该影响疫苗犹豫率的关键,医务人员应主动宣传新冠疫苗的接种注意事项、不良反应和有效性。 -
关键词:
- HIV/AIDS人群 /
- 新型冠状病毒疫苗 /
- 接种意愿 /
- 疫苗犹豫
Abstract:Objective To investigate the vaccination status and vaccination willingness of novel coronavirus in HIV/AIDS population in Yunnan.
Methods From October 2021 to June 2022, a questionnaire survey was conducted among 2180 HIV/AIDS patients in Kunming, Qujing, Yuxi, Zhaotong, Puer, Baoshan, Lincang, Honghe, Wenshan, Xishuangbanna, Dali, Dehong and Nujiang prefectures. The questionnaire included age, sex, education, nationality, education level, vaccination, adverse reactions within 7 days after the vaccination, safety of COVID-19 vaccine, awareness of effectiveness, vaccination willingness and so on. Results Among the subjects, 2109 completed 3 injections, accounting for 96.74%, and 71 were not vaccinated, accounting for 3.26%. Within 7 days of inoculation, local adverse reactions occurred in 116 cases, accounting for 5.50%, and systemic adverse reactions occurred in 56 cases, accounting for 2.66%. Injection site pain, fatigue and muscle pain accounted for the highest proportion of adverse symptoms in different sex, age and the Han nationality, while the proportion of minority adverse reactions was very low, and there was no difference among the different sex and age (P > 0.05). The main reasons for the reluctance of HIV/AIDS population to be vaccinated were(recommended by doctors) that HIV/AIDS patients could not be vaccinated (67.61%) and may have serious adverse reactions after the vaccination (19.72%). The factors affecting the vaccination were found by logistic regression analysis, whether they were worried about infecting novel coronavirus (OR = 0.121, 95%CI = 0.083~0.640, P < 0.001) and how much they knew about COVID-19 vaccine (OR = 28.932, 95%CI = 15.469~54.115, P < 0.001), safety of vaccination(OR = 13.953, 95%CI = 4.819~40.404, P < 0.001) and belief in the preventive effect of vaccine (OR = 14.017, 95%CI = 4.752~41.348, P < 0.001) were significant factors affecting vaccination. Among the 13 prefectures and cities, Dehong (20), Zhaotong (21) and Lincang (14) had the largest number of unvaccinated people. Conclusion After the mass vaccination, the rate of adverse reaction in HIV/AIDS population is low, the symptoms are mild, the correct and scientific advice and guidance from doctors and the full understanding of the harmfulness of the disease, the safety, prevention and effectiveness of the vaccine are the key to complete vaccination and put an end to vaccine hesitancy. -
Key words:
- HIV/AIDS population /
- COVID-19 vaccine /
- Vaccination willingness /
- Vaccine hesitancy
-
表 1 HIV/AIDS人群新型冠状病毒疫苗接种基本情况[n(%)/M(P25,P75]
Table 1. Basic situation of COVID-19 vaccination in HIV/AIDS population [n(%)/M(P25,P75]
项目 接种组 未接种组 Z/χ2 P 占比 2109(96.74) 71(3.26) 1406.775 < 0.001 年龄(岁) 44(38,52) 44(37,53) 0.333 0.739 性别 男 1238(58.70) 48(67.61) 2.251 0.142 女 871(41.30) 23(32.39) 民族 汉族 1738(82.41) 54(76.06) 1.895 0.205 少数民族 371(17.59) 17(23.94) 少数民族种类 佤族 63(16.98) 5(29.41) 1.480 0.830 彝族 62(16.71) 傣族 50(13.48) 6(35.29) 壮族 42(11.32) 苗族 28(7.55) 白族 23(6.20) 瑶族 18(4.85) 回族 16(4.31) 傈僳族 16(4.31) 3(17.65) 景颇族 14(3.77) 2(11.76) 哈尼族 12(3.23) 怒族 10(2.70) 独龙族 8(2.16) 布朗族 6(1.62) 1(5.88) 德昂族 2(0.54) 布依族 1(0.27) 文化程度 初中及以下 1842(87.34) 61(85.92) 0.149 0.928 中专及高中 181(8.58) 7(9.86) 大专及以上 86(4.08) 3(4.23) 表 2 HIV/AIDS人群疫苗接种7 d内不良反应(%)
Table 2. Adverse reactions of vaccination in HIV /AIDS population within 7 days (%)
项目 注射点疼痛 χ2 P 疲劳 χ2 P 肌肉疼痛 χ2 P 性别 男 40.52 1.240 0.295 23.21 0.033 1.000 14.29 4.145 0.056 女 52.59 21.43 35.71 年龄(岁) ≤ 45 53.45 1.621 0.244 32.14 3.982 0.050 21.53 0.458 0.533 > 45 39.66 12.50 28.57 民族 汉族 79.31 38.413 < 0.001 35.71 7.447 0.007 41.07 9.413 0.003 少数民族 13.79 8.93 8.93 表 3 HIV/AIDS人群疫苗认知情况和接种意愿[n(%)]
Table 3. The cognitive situation of vaccine and vaccination willingness in HIV /AIDS population [n(%)]
项目 接种组 未接种组 χ2 P 是否担心感染新冠病毒 担心 1908(90.47) 53(74.65) 19.027 < 0.001* 不担心 201(9.53) 18(25.35) 对新冠疫苗了解程度 了解一些 1844(87.43) 22(30.99) 177.526 < 0.001* 不了解 265(12.57) 49(69.01) 接种疫苗是否安全 安全 1891(89.66) 41(57.75) 69.402 < 0.001* 不安全 218(10.34) 30(42.25) 是否相信疫苗的预防作用 是 2096(99.38) 62(87.32) 99.997 < 0.001* 否 13(0.62) 9(12.68) 疫苗接种前最担心的事情 注射后无效 54(2.56) 4(5.63) 4.613 0.100 出现严重不良反应 484(22.95) 21(29.58) 诱发病情加重 1571(74.49) 46(64.79) 促使您接种疫苗的主要原因 工作需求 18(0.85) 主动选择 737(34.95) 医师建议 1138(53.96) 家人朋友建议 179(8.49) 其他 37(1.75) 不愿意接种疫苗的主要原因 疫苗本身不安全 2(2.82) 接种后有严重不良反应 14(19.72) 疫苗没有保护作用 2(2.82) HIV/AIDS患者不能接种疫苗(医师建议) 48(67.61) 有慢性病(高血压、糖尿病等)不能接种 3(4.23) 其他原因(年龄、药物过敏等) 2(2.82) *P<0.05。 表 4 HIV/AIDS人群接种意愿影响因素量化赋值
Table 4. Assignment of the influencing factor of vaccination in HIV /AIDS population
自变量 因素 赋值 X1 是否担心感染新冠病毒 担心 = 0,不担心 = 1 X2 对新冠疫苗了解程度 了解 = 0,不了解 = 1 X3 接种疫苗是否安全 安全 = 0,不安全 = 1 X4 是否相信疫苗的预防作用 是 = 0,否 = 1 表 5 影响HIV/AIDS人群疫苗接种的多因素 Logistic 回归分析
Table 5. Multivariate logistic regression analysis in associated factors of vaccination in HIV/AIDS population
因素 B SE Wald χ2 P OR OR 95% CI 是否担心感染新冠病毒 −4.484 0.640 49.053 < 0.001 0.121 0.083~0.140 对新冠疫苗了解程度 3.365 0.319 110.945 < 0.001 28.932 15.469~54.115 接种疫苗是否安全 2.636 0.542 23.607 < 0.001 13.953 4.819~40.404 是否相信疫苗的预防作用 2.640 0.552 22.883 < 0.001 14.017 4.752~41.348 -
[1] Ryan J, Malinga T. Interventions for vaccine hesitancy[J]. Curr Opin Immunol, 2021, 71(期号?): 89-91.Kelso J M. The adverse reactions to vaccines practice parameter 10 years on-what have we learned?[J]. Ann Allergy Asthma Immunol,2022,129(1):35-39. [2] Wibawa T. COVID-19 vaccine research and development: Ethical issues[J]. Trop Med Int Health,2021,26(1):14-19. doi: 10.1111/tmi.13503 [3] 王珞,范俊平,徐燕,等. 新型冠状病毒疫苗的研发现状与挑战[J]. 中华结核和呼吸杂志,2021,44(5):492-496. [4] 廖聪慧,王子晨,邓强,等. COVID-19疫苗上市后安全性及有效性的研究进展[J]. 暨南大学学报(自然科学与医学版),2021,42(5):547-556. doi: 10.11778/j.jdxb.2021.05.011 [5] Polack F P,Thomas S J,Kitchin N,et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine[J]. N Engl J Med,2020,383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [6] Fiolet T,Kherabi Y,MacDonald CJ,et al. Comparing COVID-19 vaccines for their characteristics,efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review[J]. Clin Microbiol Infect,2022,28(2):202-221. doi: 10.1016/j.cmi.2021.10.005 [7] World Health Organization. Coronavirus (COVID-19) dashboard[EB/OL]. (2022-11-28)[2022 -11-28]. https://covid19.who.int/. [8] Xia S,Duan K,Zhang Y,et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: Interim analysis of 2 randomized clinical trials[J]. JAMA,2020,324(10):951-960. doi: 10.1001/jama.2020.15543 [9] Hippisley-Cox J,Coupland CA,Mehta N,et al. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: National prospective cohort study[J]. BMJ,2021,374(5):n2244. [10] 麦迪娜·金格斯,郑碧芸,尹平. 新型冠状病毒疫苗Ⅲ期临床试验及真实世界研究[J]. 医药导报,2021,40(9):1159-1168. [11] Li M,Wang H,Tian L,et al. COVID-19 vaccine development: Milestones,lessons and prospects[J]. Signal Transduct Target Ther,2022,7(1):146. doi: 10.1038/s41392-022-00996-y [12] Meo SA,Bukhari IA,Akram J,et al. COVID-19 vaccines: Comparison of biological,pharmacological characteristics and adverse effects of Pfizer/BioNTech and moderna vaccines[J]. Eur Rev Med Pharmacol Sci,2021,25(3):1663-1669. [13] Yamamoto K. Adverse effects of COVID-19 vaccines and measures to prevent them[J]. Virol J,2022,19(1):100. doi: 10.1186/s12985-022-01831-0 [14] 杨惠洁,孙誉芳,王军志,等. 新型冠状病毒感染疫苗有效性和安全性概述[J]. 中国新药杂志,2022,31(21):2082-2089. [15] Chen Y,Xu Z,Wang P,et al. New-onset autoimmune phenomena post-COVID-19 vaccination[J]. Immunology,2022,165(4):386-401. doi: 10.1111/imm.13443 [16] Guo M,Liu X,Chen X,et al. Insights into new-onset autoimmune diseases after COVID-19 vaccination[J]. Autoimmun Rev,2023,22(7):103340. doi: 10.1016/j.autrev.2023.103340 [17] Gao Y,Yang X,Chen H,et al. A pangenome reference of 36 Chinese populations[J]. Nature,2023,619(7968):112-121. doi: 10.1038/s41586-023-06173-7 [18] 苏品璨, 何知航, 纪欣, 等. 云南九个少数民族HLA/KIR配受体对研究[C]//云南省环境科学学会, 云南省林学会, 生物多样性研究. 云南出版集团, 2021: 9. [19] 张志丹. 云南四种少数民族G6PD基因罕见突变致病性研究[D]. 北京: 北京协和医学院硕士学位论文, 2022. [20] 张信辉,孙永根,李小英,等. PIMA T淋巴细胞分析仪在少数民族地区健康人外周血CD4+T淋巴细胞正常参考值的建立[J]. 中国艾滋病性病,2018,24(11):1085-1087,1096. [21] Kricorian K,Civen R,Equils O. COVID-19 vaccine hesitancy: Misinformation and perceptions of vaccine safety[J]. Hum Vaccin Immunother,2022,18(1):1950504. doi: 10.1080/21645515.2021.1950504 [22] Wiysonge CS,Ndwandwe D,Ryan J,et al. Vaccine hesitancy in the era of COVID-19: Could lessons from the past help in divining the future?[J]. Hum Vaccin Immunother,2022,18(1):1-3. [23] Lee W S,Wheatley A K,Kent S J,et al. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies[J]. Nat Microbiol,2020,5(10):1185-1191. doi: 10.1038/s41564-020-00789-5 [24] Vinhaes CL,Araujo-Pereira M,Tibúrcio R,et al. Systemic inflammation associated with immune reconstitution inflammatory syndrome in persons living with HIV[J]. Life (Basel),2021,11(1):65. doi: 10.3390/life11010065 [25] Giacomelli A,Pezzati L,Rusconi S. The crosstalk between antiretrovirals pharmacology and HIV drug resistance[J]. Expert Rev Clin Pharmacol,2020,13(7):739-760. doi: 10.1080/17512433.2020.1782737 -





 下载:
下载: