A Meta-analysis of the Risk of Secondary Infection of Tocilizumab in the Treatment of COVID-19
-
摘要:
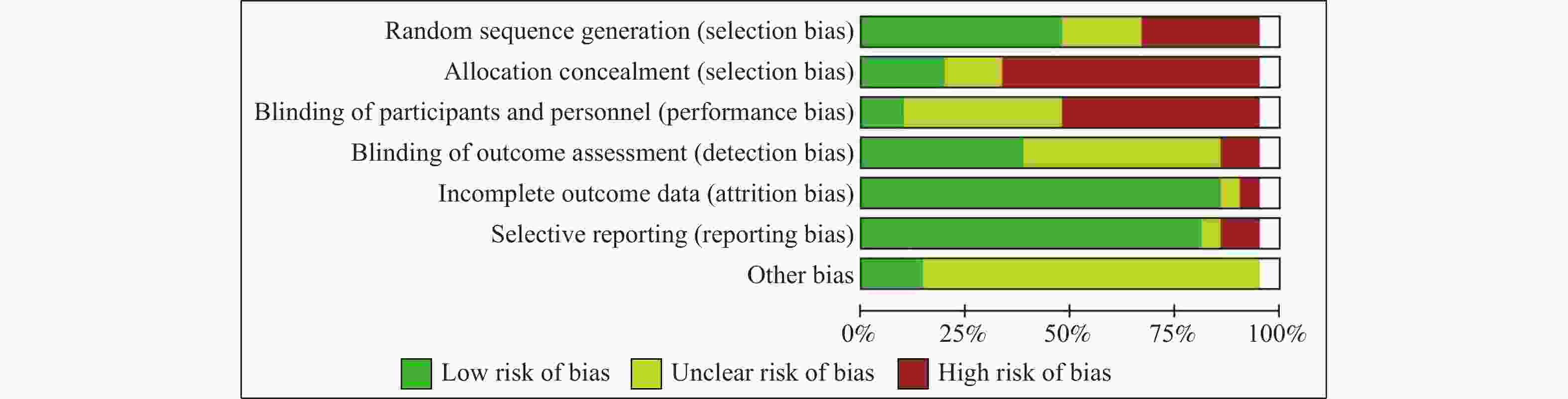
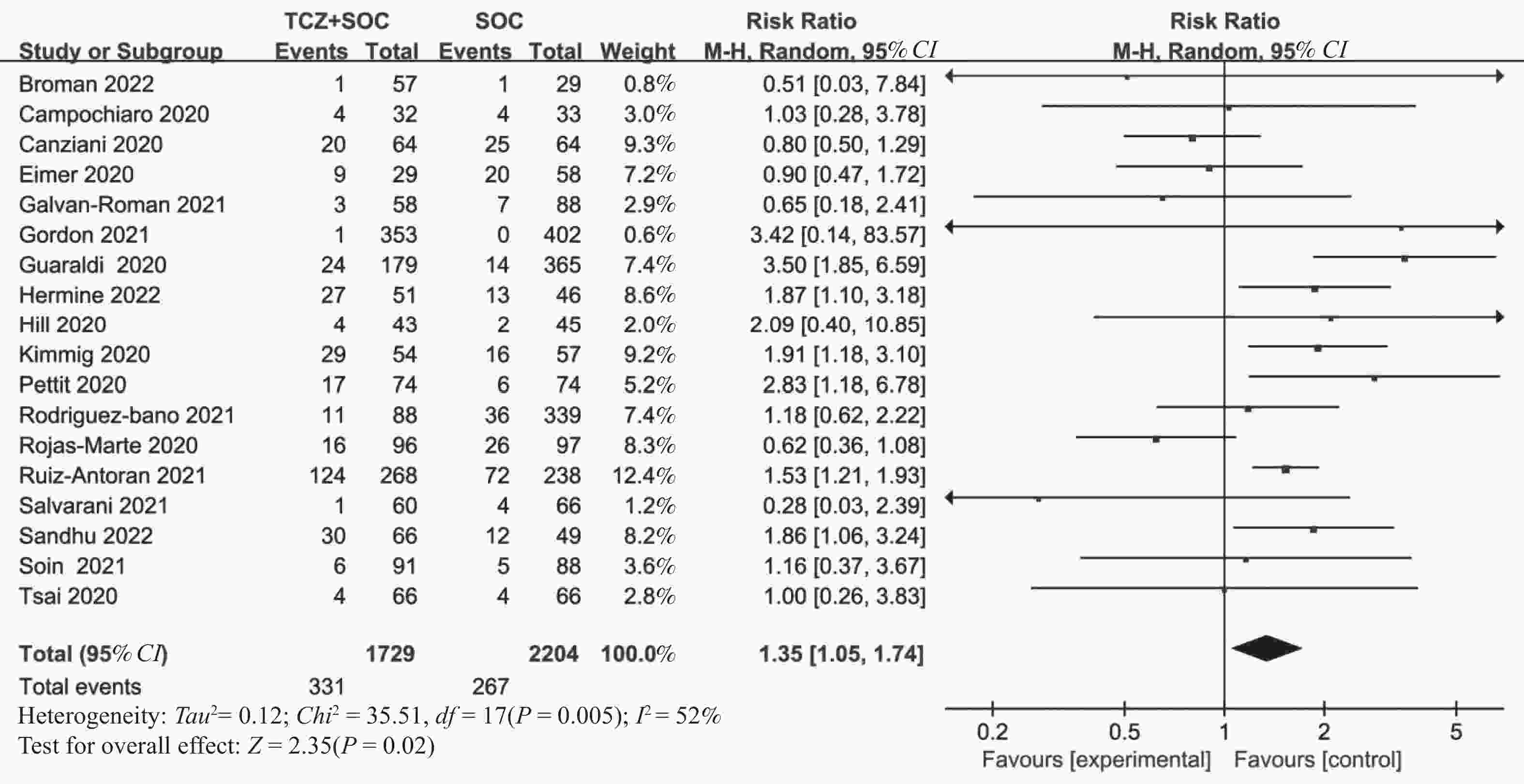
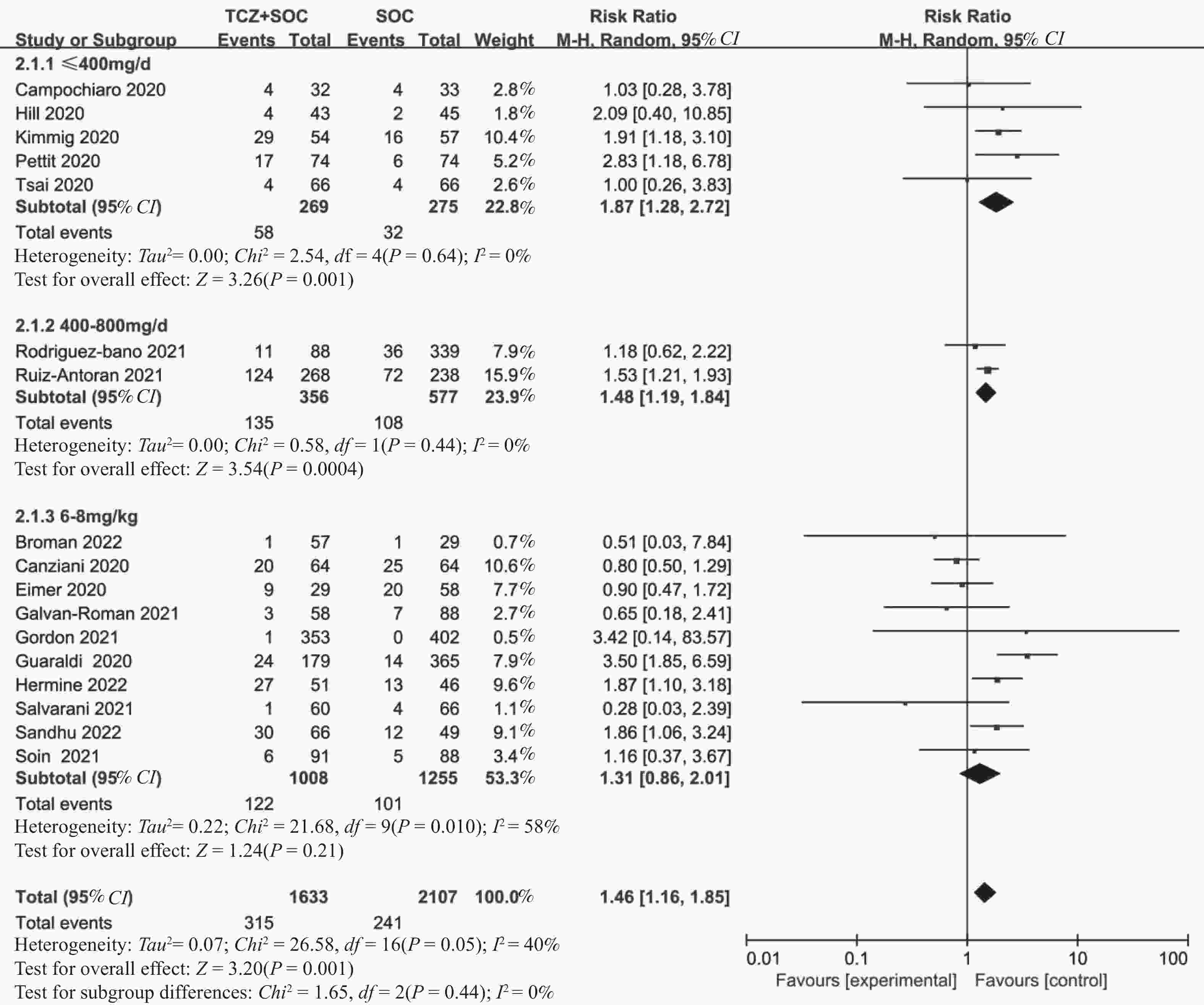
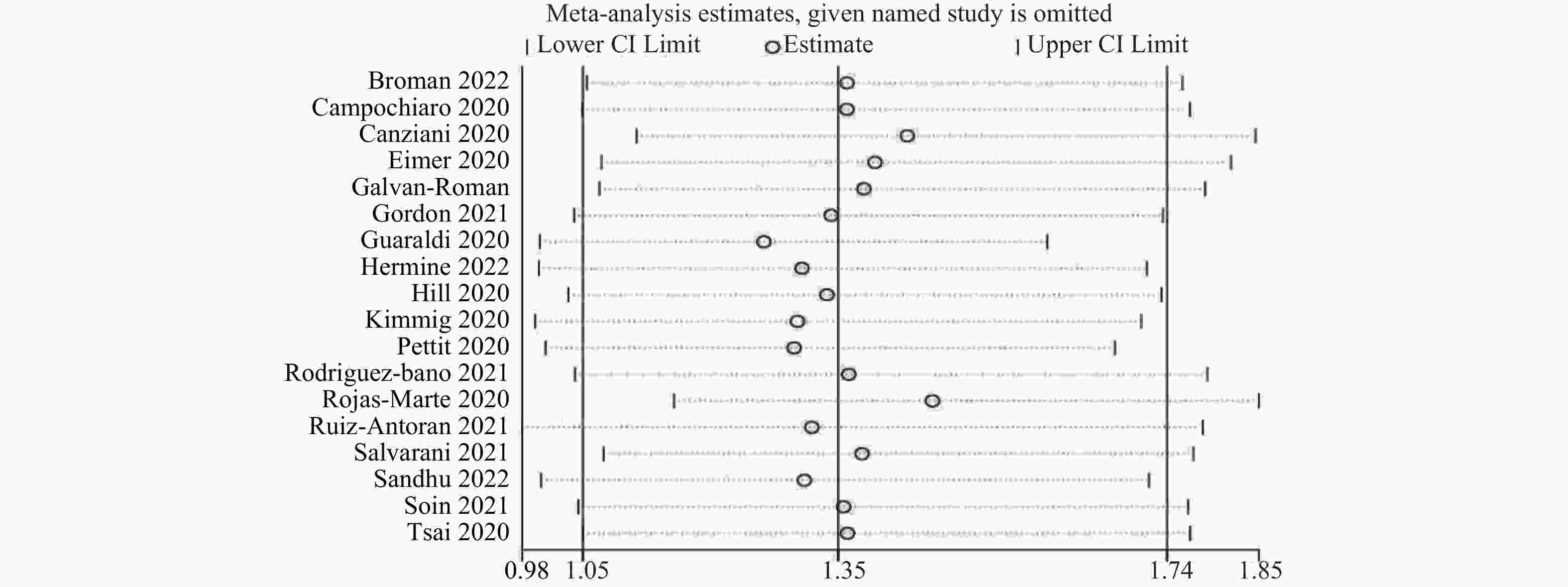
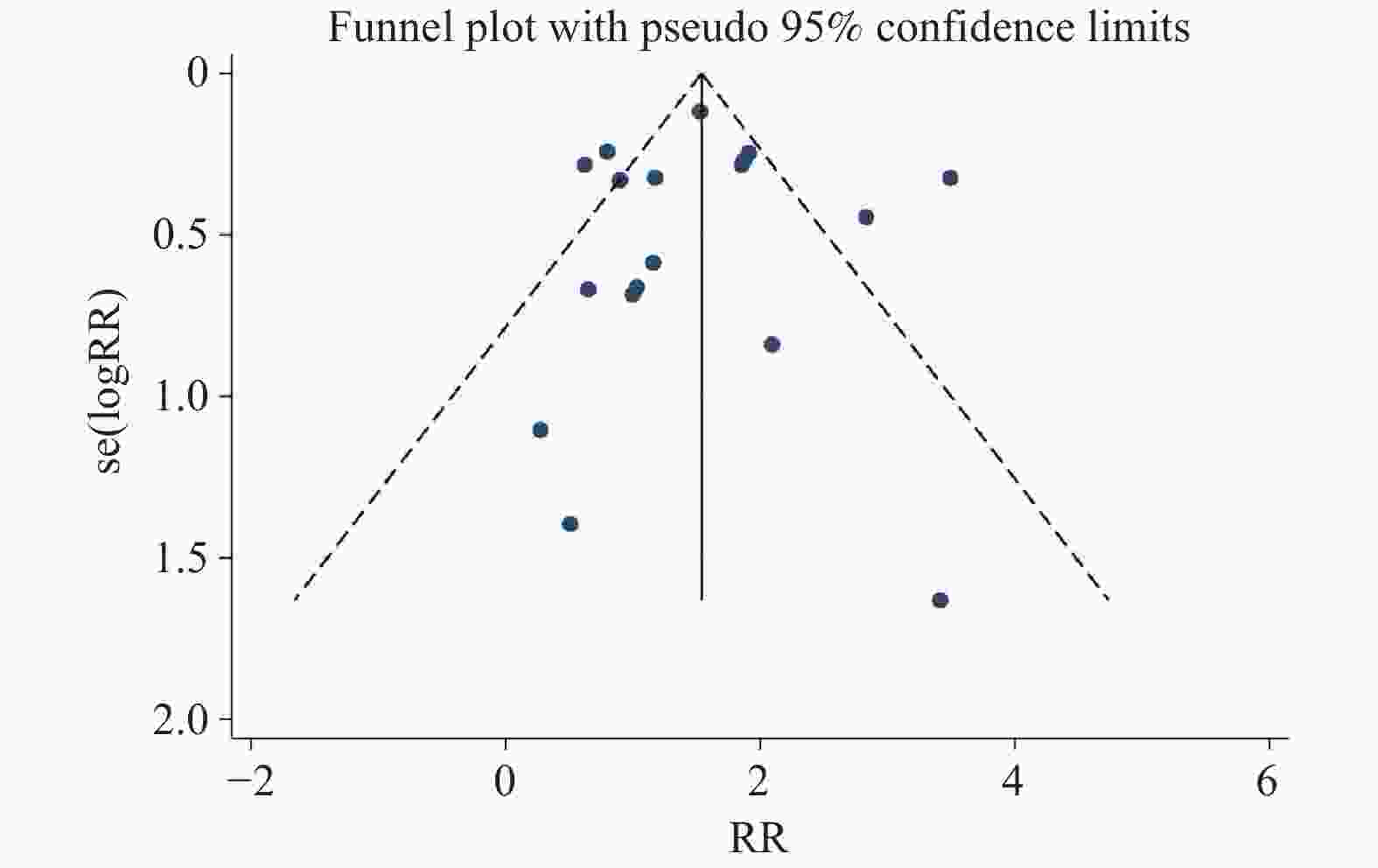
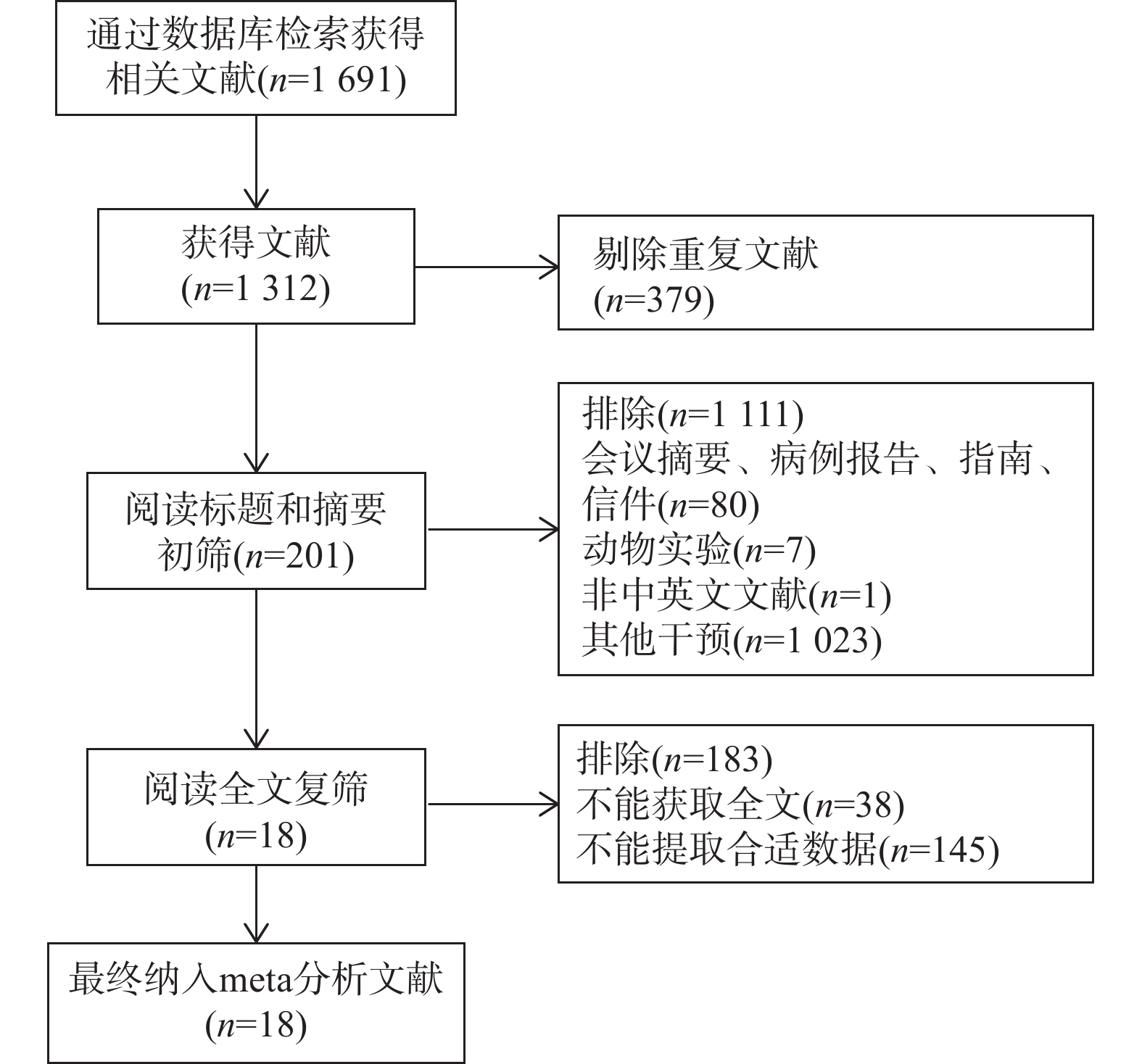
目的 通过Meta分析评估托珠单抗(tocilizumab,TCZ)治疗新型冠状病毒感染(corona virus disease 2019,COVID-19)导致的继发感染风险,为托珠单抗在COVID-19患者中应用的安全性提供循证依据。 方法 在The Cochrane Library、PubMed、Web of Science、中国知网、中国生物医学文献数据库以及万方数据库中检索了2019年12月19日至2022年12月30日期间使用托珠单抗治疗COVID-19患者的相关研究,筛选并提取文献中发生继发感染的数据,利用RevMan 5.4.1进行Meta分析。 结果 共筛选了 1691 篇参考文献,纳入18项研究,涉及3933 名患者。托珠单抗+标准治疗组继发感染发生率为19.14%(331/1729 ),标准治疗组继发感染发生率为12.11%(267/2204 )。Meta分析结果显示,托珠单抗+标准治疗组继发感染发生率高于标准治疗组[RR = 1.35,95%CI (1.05,1.74),P = 0.02]。亚组分析显示,使用不同剂量的托珠单抗发生继发感染的风险不同。托珠单抗给药剂量为400~800 mg/d的亚组继发感染发生率明显高于标准治疗组,差异具有统计学意义[RR = 1.48,95%CI (1.19,1.84),P =0.0004 ];≤400 mg/d继发感染发生率也显著高于标准治疗组,差异具有统计学意义[RR = 1.87,95%CI (1.28,2.72),P = 0.001];托珠单抗给药剂量为6~8 mg/kg亚组与标准治疗组比较差异无统计学意义。结论 与标准治疗相比,托珠单抗可能增加COVID-19患者发生继发感染的风险,临床给药前应仔细评估使用托珠单抗治疗的利益和风险。但是,目前仍需要更多大样本、高质量的研究来进一步评估。 Abstract:Objective Meta-analysis was conducted to assess the risk of secondary infection caused by tocilizumab (TCZ) in the treatment of Corona Virus Disease 2019 (COVID-19), in order to provide an evidence-based basis for the safety of tocilizumab in patients with COVID-19. Methods Cochrane Library, PubMed, Web of Science, CNKI, SinoMed and Wanfang databases were searched in computer to collect randomized controlled trial and cohort study of treating COVID-19 with tocilizumab from December 19, 2019 to December 30, 2022. A meta-analysis of the results of each study was performed using RevMan 5.4.1 software. Results A total of 1691 references were screened and eighteen studies involving3933 patients were included. The incidence of secondary infection in the tocilizumab with the standard treatment group and standard treatment group was 19.14% (331/1729 ) and 12.11% (267/2204 ), respectively. Meta-analysis showed that the tocilizumab + standard treatment group had a higher incidence of secondary infection than the standard treatment group [RR = 1.35, 95%CI (1.05, 1.74), P = 0.02]. The results of the subgroup analysis showed that the risk of secondary infection with different doses of tocilizumab was different. The incidence of secondary infection was significantly higher in the subgroup with doses of 400~800 mg/d tocilizumab than in the standard care group [RR = 1.48, 95%CI (1.19, 1.84), P =0.0004 ]. The incidence of secondary infection in subgroups with doses of ≤400 mg/d tocilizumab was also significantly higher than that in the standard treatment group [RR = 1.87, 95%CI (1.28, 2.72), P = 0.001]. However, there was no statistical significance between the subgroup 6~8 mg/kg tocilizumab and the standard treatment group.Conclusions Tocilizumab may increase the risk of secondary infection in patients with COVID-19 compared with standard treatment, and the benefits and risks of tocilizumab should be carefully evaluated before clinical administration. Moreover, large and high-quality studies are needed for further evaluation. -
Key words:
- Tocilizumab /
- COVID-19 /
- Secondary infection /
- Meta-analysis
-
表 1 纳入文献的基本特征(n)
Table 1. Basic characteristics of the included studies (n)
第一作者 年份 国家 COVID-19
患者特点年龄(岁)
(TCZ/SOC)总例数 托珠
单抗组对照组 剂量 继发感染 随访
时间(/d)托珠
单抗组对照
组Broman[5] 2022 芬兰 全身炎症住院患者 58/59 86 57 29 8 mg/kg 1 1 28 Campochiaro[6] 2020 意大利 重症患者 65/60 65 32 33 400 mg/d 4 4 28 Canziani[7] 2020 意大利 重症患者 63/64 128 64 64 8 mg/kg 20 25 30 Eimer[8] 2020 瑞典 重症患者 56/56 87 29 58 8 mg/kg 9 20 30 Galván-Román[9] 2021 西班牙 重症患者 61/64 146 58 88 8 mg/kg 3 7 12 Gordon[10] 2021 多中心 危重患者 62/61 755 353 402 8 mg/kg 1 0 69 Guaraldi[11] 2020 意大利 重症患者 67/69 544 179 365 8 mg/kg 24 14 / Hermine[12] 2022 法国 中重、危重度患者 43/57 97 51 46 8 mg/kg 27 13 95 Hill[13] 2020 美国 重症患者 / 88 43 45 400 mg/d 4 2 28 Kimmig[14] 2020 美国 危重患者 / 111 54 57 400 mg/d 29 16 / Pettit [15] 2020 美国 / 66/65 148 74 74 400 mg/d 17 6 58 Rodríguez-Baño[16] 2021 西班牙 高炎症状态患者 66/69 427 88 339 400~800 mg/d 11 36 21 Rojas-Marte[17] 2020 美国 重症患者 58.8/62 193 96 97 / 16 26 / Ruiz-Antorán[18] 2021 西班牙 重症患者 65/71.3 506 268 238 400~800 mg/d 124 72 28 Salvarani[19] 2021 意大利 重症患者 61.5/60 126 60 66 8 mg/kg 1 4 30 Sandhu[20] 2022 / 重症患者 68.2/63.9 115 66 49 8 mg/kg 30 12 / Soin[21] 2021 印度 中、重度患者 55/53 179 91 88 6 mg/kg 6 5 30 Tsai[22] 2020 美国 重症患者 62/61 132 66 66 400 mg/d 4 4 / 注: TCZ:托珠单抗; SOC:标准治疗。 -
[1] Ragab D,Eldin H S,Taeimah M,et al. The COVID-19 cytokine storm;what we know so far[J]. Front Immunol,2020,11:1446. doi: 10.3389/fimmu.2020.01446 [2] Liu B,Lim,Zhou Z,et al. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)?[J]. J Autoimmun,2020,111:102452. doi: 10.1016/j.jaut.2020.102452 [3] Tang Y,Liu J,Zhang D,et al. Cytokine storm in COVID-19: The current evidence and treatment strategies[J]. Front Immunol,2020,11:1708. doi: 10.3389/fimmu.2020.01708 [4] Yang X,Yu Y,Xu J,et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan,China: A single-centered,retrospective,observational study[J]. Lancet Respir Med,2020,8(5):475-481. doi: 10.1016/S2213-2600(20)30079-5 [5] Broman N,Feuth T,Vuorinen T,et al. Early administration of tocilizumab in hospitalized COVID-19 patients with elevated inflammatory markers;COVIDSTORM-a prospective,randomized,single-centre,open-label study[J]. Clin Microbiol Infect,2022,28(6):844-851. doi: 10.1016/j.cmi.2022.02.027 [6] Campochiaro C,Della-Torre E,Cavalli G,et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: A single-centre retrospective cohort study[J]. Eur J Intern Med,2020,76:43-49. doi: 10.1016/j.ejim.2020.05.021 [7] Canziani L M,Trovati S,Brunetta E,et al. Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: A retrospective case-control survival analysis of 128 patients[J]. J Autoimmun,2020,114:102511. doi: 10.1016/j.jaut.2020.102511 [8] Eimer J,Vesterbacka J,Svensson A K,et al. Tocilizumab shortens time on mechanical ventilation and length of hospital stay in patients with severe COVID-19: A retrospective cohort study[J]. J Intern Med,2021,289(3):434-436. doi: 10.1111/joim.13162 [9] Galv á n-Rom á n J M,Rodr í guez-Garc í a S C,Roy-Vallejo E,et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: An observational study[J]. J Allergy Clin Immunol,2021,147(1):72-80.e8. doi: 10.1016/j.jaci.2020.09.018 [10] Gordon A C,Angus D C,Derde L P G,et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19[J]. N Engl J Med,2021,385(12):1147-1149. doi: 10.1056/NEJMc2108482 [11] Guaraldi G,Meschiari M,Cozzi-Lepri A,et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study[J]. Lancet Rheumatol,2020,2(8):e474-e484. doi: 10.1016/S2665-9913(20)30173-9 [12] Hermine O,Mariette X,Porcher R,et al. Effect of interleukin-6 receptor antagonists in critically ill adult patients with COVID-19 pneumonia: Two randomised controlled trials of the CORIMUNO-19 Collaborative Group[J]. Eur Respir J,2022,60(2):2102523. doi: 10.1183/13993003.02523-2021 [13] Hill J A,Menon M P,Dhanireddy S,et al. Tocilizumab in hospitalized patients with COVID-19: Clinical outcomes,inflammatory marker kinetics,and safety[J]. J Med Virol,2021,93(4):2270-2280. doi: 10.1002/jmv.26674 [14] Kimmig L M,Wu D,Gold M,et al. IL-6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections[J]. Front Med(Lausanne),2020,7:583897. [15] Pettit N N,Nguyen C T,Mutlu G M,et al. Late onset infectious complications and safety of tocilizumab in the management of COVID-19[J]. J Med Virol,2021,93(3):1459-1464. doi: 10.1002/jmv.26429 [16] Rodríguez-Baño J,Pachón J,Carratalà J,et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: A multicentre cohort study (SAM-COVID-19)[J]. Clin Microbiol Infect,2021,27(2):244-252. doi: 10.1016/j.cmi.2020.08.010 [17] Rojas-Marte G,Khalid M,Mukhtar O,et al. Outcomes in patients with severe COVID-19 disease treated with tocilizumab: A case-controlled study[J]. QJM,2020,113(8):546-550. doi: 10.1093/qjmed/hcaa206 [18] Ruiz-Antor á n B,Sancho-L ó pez A,Torres F,et al. Combination of tocilizumab and steroids to improve mortality in patients with severe COVID-19 infection: A Spanish,multicenter,cohort study[J]. Infect Dis Ther,2021,10(1):347-362. doi: 10.1007/s40121-020-00373-8 [19] Salvarani C,Dolci G,Massari M,et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: A randomized clinical trial[J]. JAMA Intern Med,2021,181(1):24-31. doi: 10.1001/jamainternmed.2020.6615 [20] Sandhu G,Piraino S T,Piticaru J. Secondary infection risk in patients with severe COVID-19 pneumonia treated with tocilizumab[J]. Am J Ther,2022,29(3):e275-e278. doi: 10.1097/MJT.0000000000001487 [21] Soin A S,Kumar K,Choudhary N S,et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): An open-label,multicentre,randomised,controlled,phase 3 trial[J]. Lancet Respir Med,2021,9(5):511-521. doi: 10.1016/S2213-2600(21)00081-3 [22] Tsai A,Diawara O,Nahass R G,et al. Impact of tocilizumab administration on mortality in severe COVID-19[J]. Sci Rep,2020,10(1):19131. doi: 10.1038/s41598-020-76187-y [23] Abidi E,El Nekidy W S,Alefishat E,et al. Tocilizumab and COVID-19: Timing of administration and efficacy[J]. Front Pharmacol,2022,13:825749. doi: 10.3389/fphar.2022.825749 [24] Kopf M,Baumann H,Freer G,et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice[J]. Nature,1994,368(6469):339-342. doi: 10.1038/368339a0 [25] Cai S,Sun W,Li M,et al. A complex COVID-19 case with rheumatoid arthritis treated with tocilizumab[J]. Clin Rheumatol,2020,39(9):2797-2802. doi: 10.1007/s10067-020-05234-w [26] Lef è vre C,Plocque A,Tran M,et al. Should we interfere with the interleukin-6 receptor during COVID-19: What do we know?[J]. Rev Mal Respir,2023,40(1):24-37. doi: 10.1016/j.rmr.2022.11.085 [27] Narazaki M,Kishimoto T. The two-faced cytokine IL-6 in host defense and diseases[J]. Int J Mol Sci,2018,19(11):3528. doi: 10.3390/ijms19113528 [28] Lang V R,Englbrecht M,Rech J,et al. Risk of infections in rheumatoid arthritis patients treated with tocilizumab[J]. Rheumatology (Oxford),2012,51(5):852-857. doi: 10.1093/rheumatology/ker223 [29] Pawar A,Desai R J,Solomon D H,et al. Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: A multidatabase cohort study[J]. Ann Rheum Dis,2019,78(4):456-464. doi: 10.1136/annrheumdis-2018-214367 [30] Davis J S,Ferreira D,Paige E,et al. Infectious complications of biological and small molecule targeted immunomodulatory therapies[J]. Clin Microbiol Rev,2020,33(3):e00035-19. [31] Douedi S,Chaudhri M,Miskoff J. Anti-interleukin-6 monoclonal antibody for cytokine storm in COVID-19[J]. Ann Thorac Med,2020,15(3):171-173. doi: 10.4103/atm.ATM_286_20 -






 下载:
下载: